Quantum jumps: How Niels Bohr’s idea changed the world
- Niels Bohr’s atom was a truly revolutionary idea, mixing old and new physics concepts.
- In some ways, an atom resembles the solar system; in other ways, it behaves rather bizarrely.
- Bohr realized that the world of the very small demanded a new way of thinking.
The word quantum is everywhere, and along with it the term quantum jumps. Last week we discussed Max Planck’s pioneering idea that atoms might emit and absorb energy in discrete quantities, always multiples of the same amount. These small chunks of radiation got the name quantum.
This week we move forward to another key idea in the quantum revolution: Niels Bohr’s 1913 model of the atom, which gave us quantum jumps. If Planck’s idea took courage and a great deal of imagination, Bohr’s was a massive feat of bravado. Somehow Bohr put a bunch of new ideas into a bag, mixed them with old concepts from classical physics, and came up with the notion of quantized orbits in atoms. That the model held is nothing short of amazing. Bohr saw what no one could see at the time: that atoms are nothing like people had thought for at least 2,000 years. In fact, they are like nothing anyone could have imagined at all. Except Bohr, I suppose.
A revolution from the simplest particle
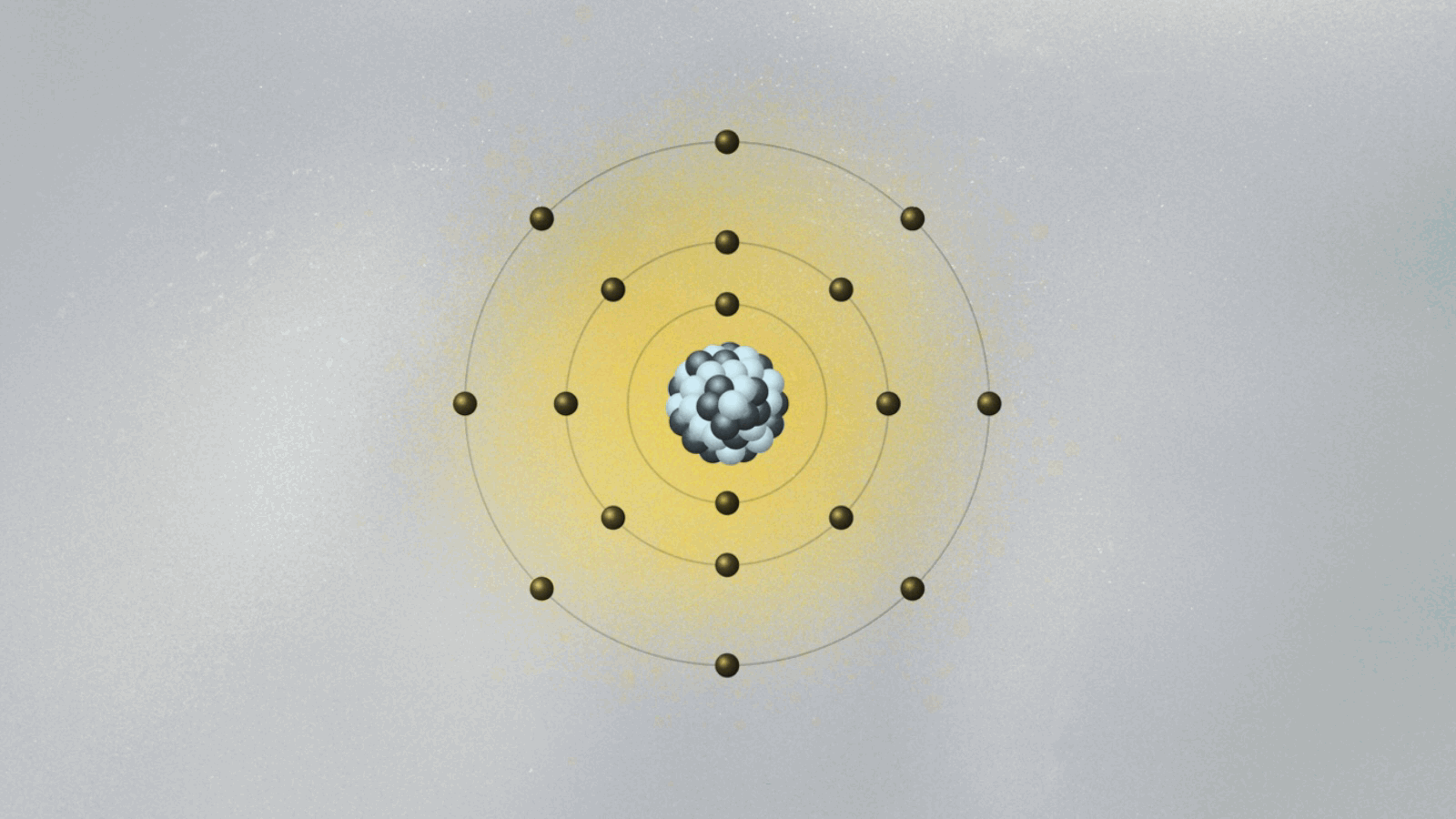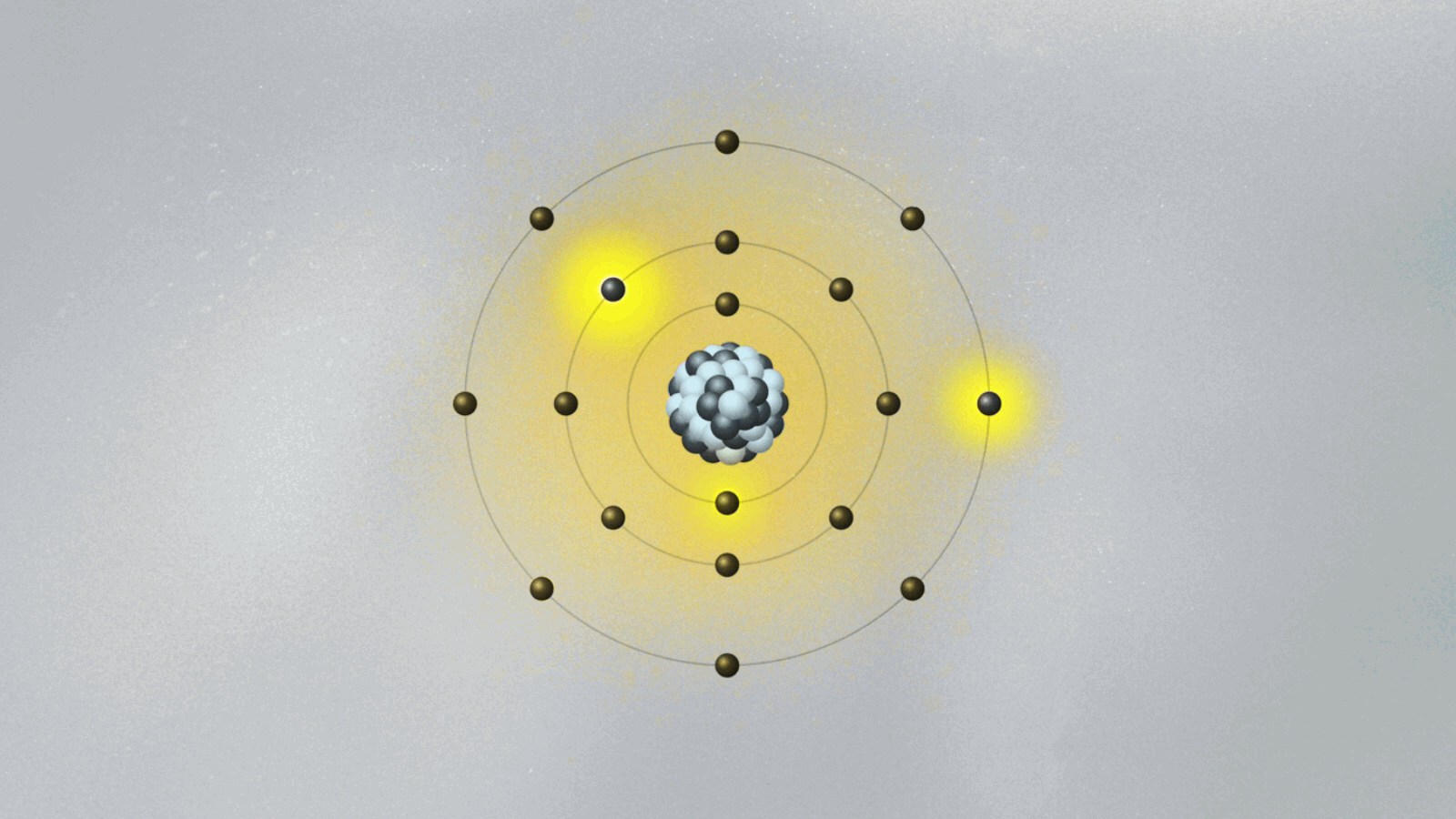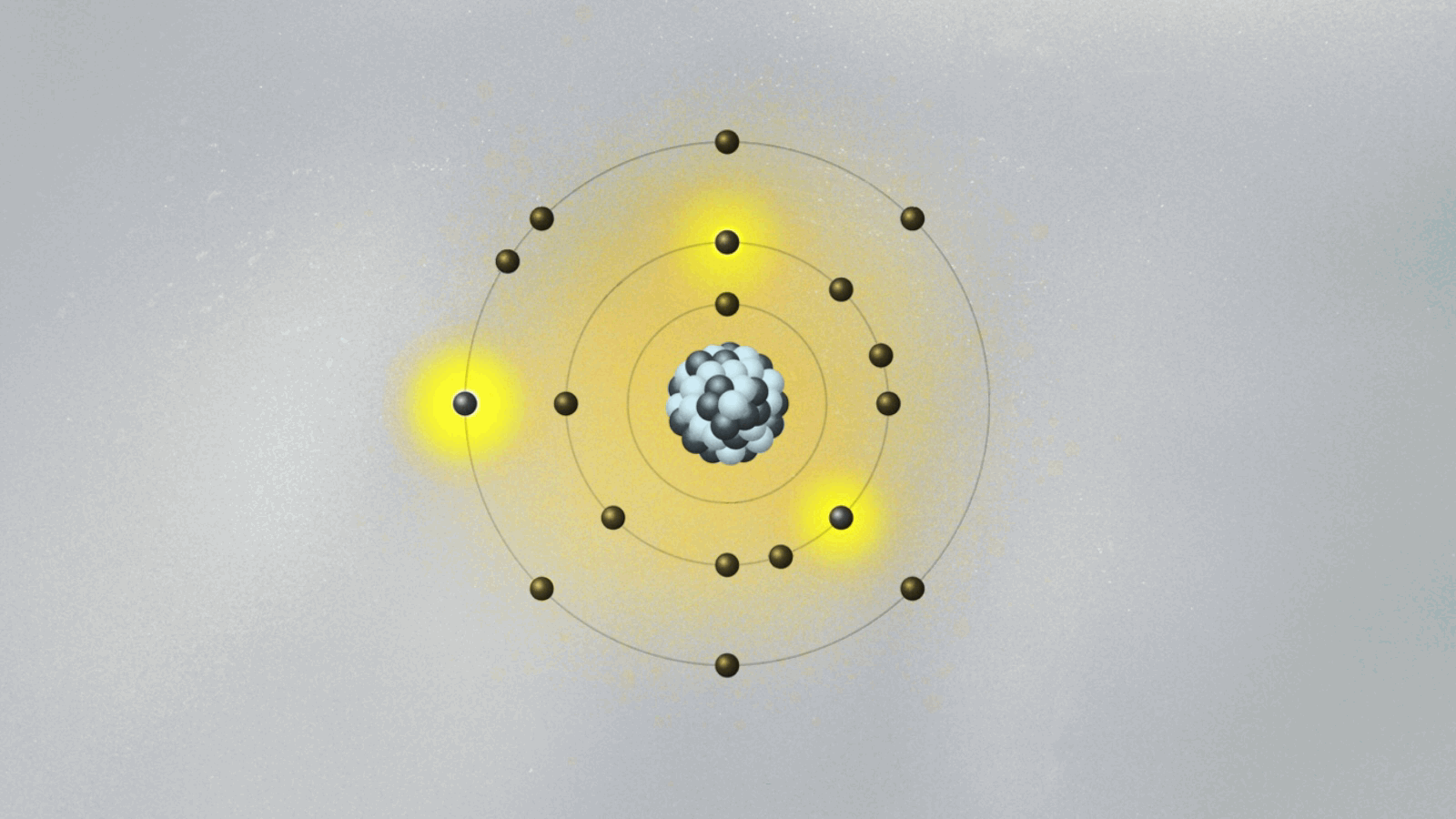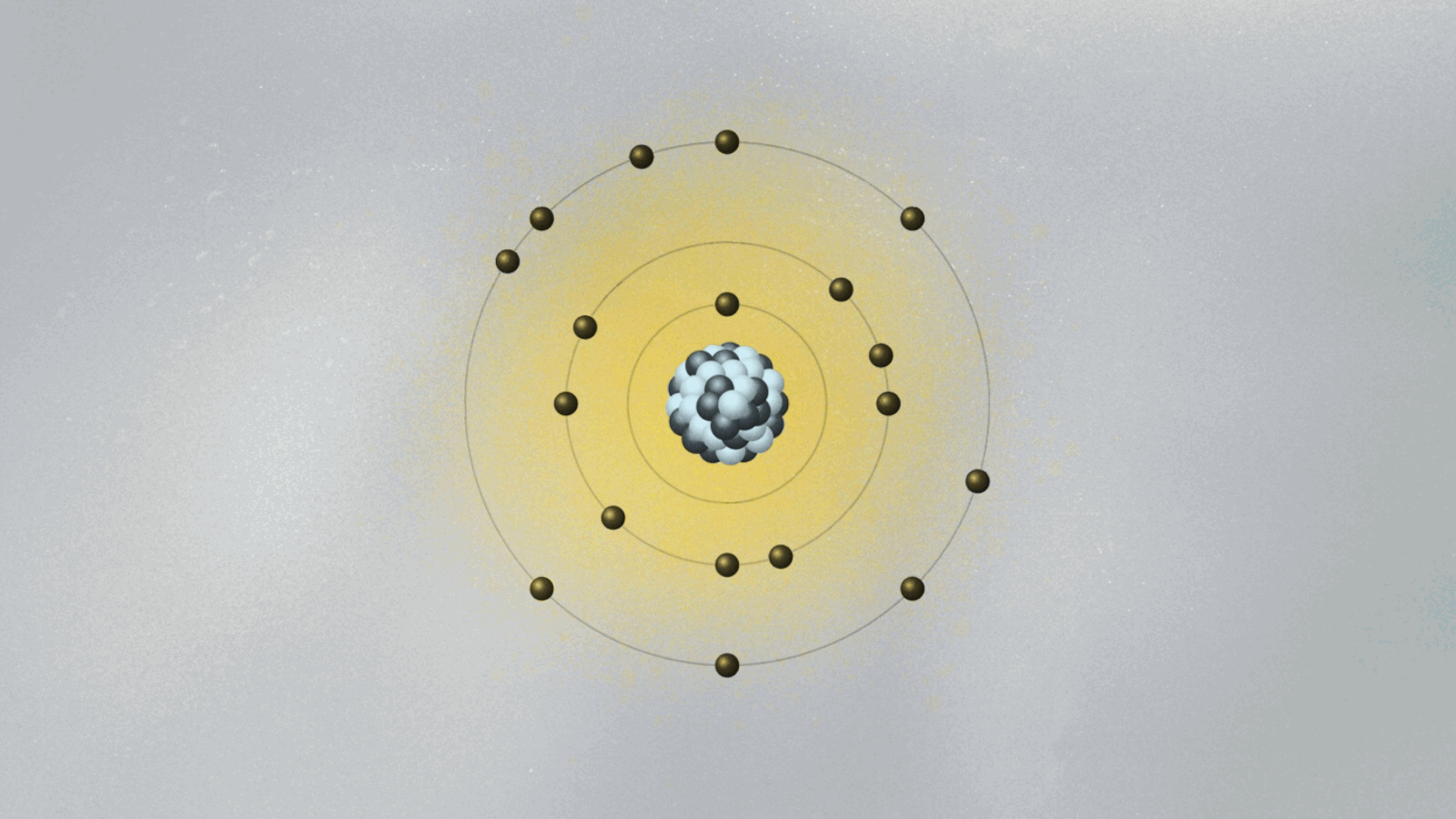
Bohr’s model of the atom is kind of crazy. His collage of ideas mixing old and new concepts was the fruit of Bohr’s amazing intuition. Looking only at hydrogen, the simplest of all atoms, Bohr formed the image of a miniature solar system, with a proton in the center and the electron circling around it.
Following the physicist’s way of doing things, he wanted to explain some of his observed data with the simplest possible model. But there was a problem. The electron, being negatively charged, is attracted to the proton, which is positive. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. As it circled the proton, it would radiate away its energy and fall in. No orbit would be stable, and atoms could not exist. Clearly, something new and revolutionary was needed. The solar system could only go so far as an analogy.
To salvage the atom, Bohr had to invent new rules that clashed with classical physics. He bravely suggested the implausible: What if the electron could only circle the nucleus in certain orbits, separated from each other in space like the steps of a ladder or the layers of an onion? Just like you can’t stand between steps, the electron can’t stay anywhere between two orbits. It can only jump from one orbit to another, the same way we can jump between steps. Bohr had just described quantum jumps.
Quantized momentum
But how are these quantum orbits determined? Again, we will bow to Bohr’s amazing intuition. But first, a foray into angular momentum.
If electrons circle protons, they have what we call angular momentum, a quantity that measures the intensity and orientation of circular motions. If you tie a rock to a string and spin it, it will have angular momentum: The faster you spin, the longer the string, or the heavier the rock, the greater this momentum is. If nothing changes in the spin velocity or the length of the string, angular momentum is conserved. In practice, it never is conserved for rotating rocks because of friction. When a whirling ice skater spins up by bringing her stretched arms to her chest, she is using her nearly conserved angular momentum: Shorter arms and more spin gives the same angular momentum as longer arms and slower spin.
Bohr suggested that the electron’s angular momentum should be quantized. In other words, it should only have certain values, given by integers (n = 1, 2, 3…). If L is the electron’s orbital angular momentum, Bohr’s formula reads, L = nh/2π, where h is the famous Planck constant we explained in last week’s essay. A quantized angular momentum means the electron’s orbits are separated in space like the steps of a ladder. The electron could go from one orbit (say the n = 2 orbit) to another (say, n = 3) either by jumping down and closer to the proton, or by jumping up and farther away.
Colorful quantum fingerprints
Bohr’s brilliant combination of concepts from classical physics with the brand-new quantum physics yielded a hybrid model of the atom. The world of the very small, he realized, asked for a new way of thinking about matter and its properties.
In the process, Bohr solved an old mystery in physics regarding the colors a chemical element emits when it is heated up, known as its emission spectrum. The strong yellow in sodium lamps is a familiar example of the dominant color in an emission spectrum. It turns out that each chemical element, from hydrogen to uranium, has its very own spectrum, characterized by a distinctive set of colors. They are an element’s spectral fingerprints. Scientists in the 19th century knew chemical spectra existed, but no one knew why. Bohr suggested that when an electron jumps between orbits it either emits or absorbs a chunk of light. These quantities of light are called photons, and they are Einstein’s key contribution to quantum physics — a contribution we will explore in this series soon.
Since the negative electron is attracted by the positive nucleus, it needs energy to jump to a higher orbit. This energy is acquired by absorbing a photon. This is the basis of the absorption spectrum, and you do the same thing every time you climb a step on a ladder. Gravity wants to hold you down, but you use the energy stored in your muscles to move up.
On the other hand, the emission spectrum of an element consists of the photons (or radiation) that electrons give off when they jump from higher orbits to lower ones. The photons carry away the angular momentum the electron loses as it jumps down. Bohr suggested that the energy of the emitted photons matches the energy difference between the two orbits.
And why do different elements have different emission spectra? Each atom has a unique number of protons in its nucleus, so its electrons are attracted by specific intensities. Each allowed orbit for each atom will have its own, specific energy. When the electron jumps between two orbits, the photon emitted will have that precise energy and no other. Back to the ladder analogy, it is as if each chemical element has its own ladder, with steps built at different distances from one another.
With this, Bohr explained the emission spectrum of hydrogen, a triumph of his hybrid model. And what happens when the electron is at the lowest level, n = 1? Well, Bohr suggests that this is the lowest it can get. He doesn’t know how, but the electron is stuck there. It does not crash down into the nucleus. His pupil, Werner Heisenberg, will give the answer some 13 years later: the Uncertainty Principle. But that’s a story for another week.