Will protons last forever? Why scientists are searching for signs of decay

- The Universe’s stability over billions of years hinges on the fact that protons, the building blocks of matter, do not decay—a surprising phenomenon given that decay is common in nature.
- This stability is thought to be due to “conservation laws” that prevent particles like protons from breaking down.
- Although experiments have yet to detect proton decay, scientists continue to search, as discovering it would reveal new insights into the fundamental laws of nature.
The Universe seems to be eternal — or close enough. For nearly 14 billion years, stars have lived and died, galaxies have spun, and matter has moved in stately ways throughout the cosmos. That might not seem remarkable at first — until you realize that this stability requires ordinary matter not to decay.
And that’s not normal. Decay is common. Ask any homeowner: Just minutes after you take possession of your house, it can seem like you’re stuck in an endless cycle of fixing things that break. And even radioactive materials eventually decay away. So, what keeps the matter of the Universe stable over cosmic time scales? Scientists don’t know the answer to that, and they have built very sensitive detectors to search for proton decay. So far, no proton decay has been observed, which certainly seems to go against the natural order of things.
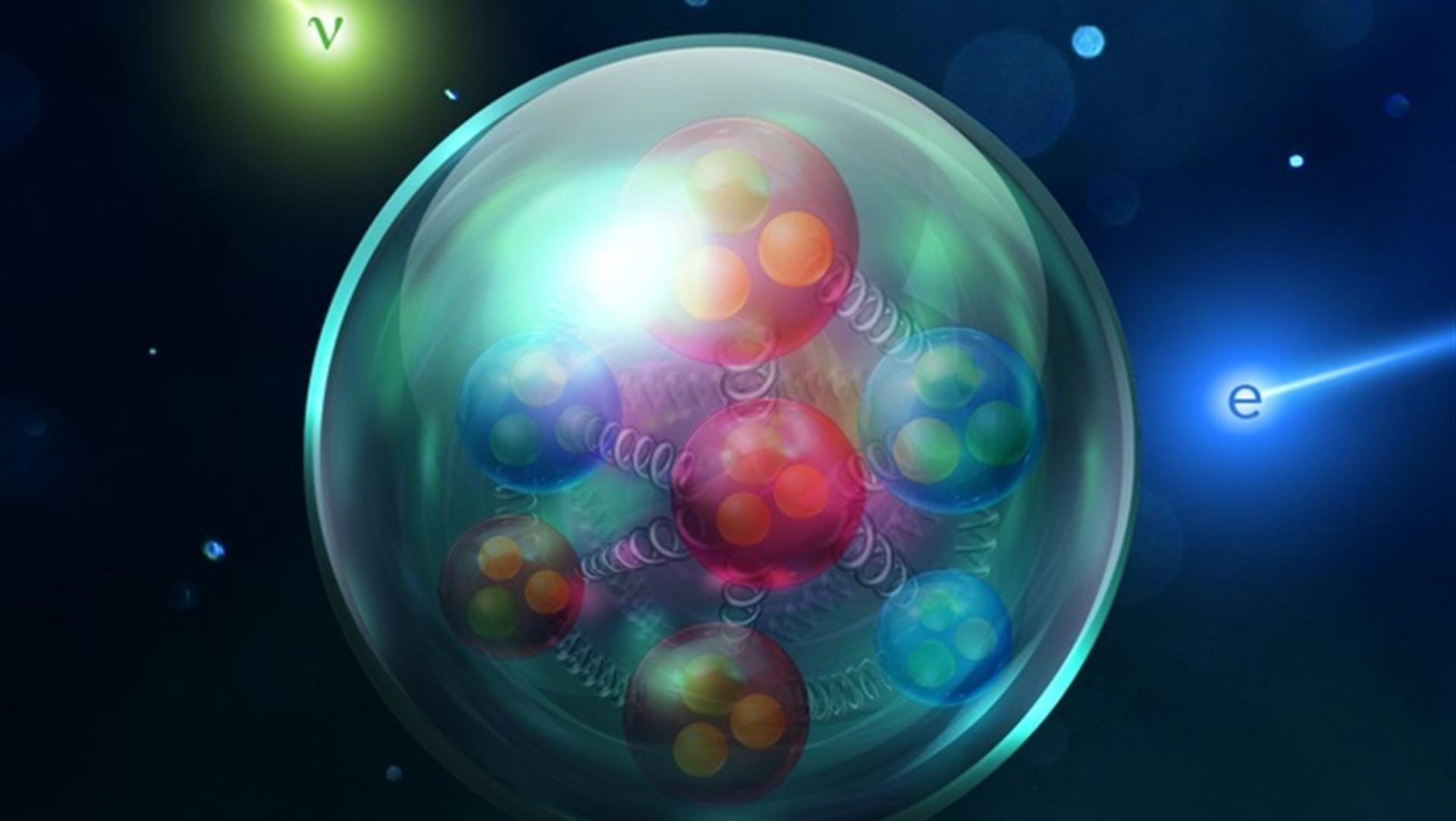
To get a handle on why protons might be stable, we need to understand what matter is made of. Basic chemistry will tell you that matter is made of atoms, but atoms themselves are made of even smaller components: protons, neutrons, and electrons. And, as it turns out, protons and neutrons are made of even smaller objects, called quarks. (We’ll get to quarks a bit later.)
On the scale of atomic and nuclear matter, what is important is that protons, neutrons, and electrons are stable. This is strictly true for protons and electrons, though not for neutrons. Although neutrons buried inside the nucleus of atoms can live forever, that’s not true for isolated neutrons. If you could extract a neutron from the center of an atom and put it in an otherwise empty bottle, the neutron would decay in just under fifteen minutes, which is far shorter than the 14-billion-year lifetime of the Universe.
Conservation laws
What saves protons and electrons from decay is a combination of so-called “conservation laws.” The term “conservation law” simply means that there is a property that never changes, no matter what.
We can use ordinary Legos to illustrate what the term means. Suppose your child has a set of 10,000 Lego pieces. As any parent knows, children can make countless things out of Legos: a building, a bridge, or, for a sufficiently artistic child, a statue of a duck. The child can use some of the pieces in their artwork or all of them; however, no matter what the child makes, what remains the same is that they still have 10,000 Lego pieces. The number of pieces is conserved.
For particles like protons, neutrons, and electrons, several conserved properties determine whether the particles are stable or not. These are energy, electric charge, and the more obscure baryon and lepton numbers.
Energy is a measure of motion, like a thrown ball, or of potential, like a coiled spring. However, because of Einstein’s most famous equation E = mc2, it is also a measure of mass. Indeed, for an isolated and stationary particle, the mass of a particle is its entire energy. Within the context of particle decay, energy conservation means that a subatomic particle can decay into a lighter particle, but not the other way around. If a heavy particle decays into a light particle, the amount of energy stored in mass gets smaller; however, the leftover energy can cause the daughter particle to move. Under this scenario, the total amount of energy is unchanged after the decay. In contrast, if a lighter particle were to decay into a heavier one, there would be more mass – and therefore more energy – after the decay than before. This would change the amount of energy, which would violate the principle of conservation of energy.
Electric charge is another conserved quantity. The proton has an electric charge of +1. If a proton decays, the sum of the electric charges of all the decay products will also have to add up to +1. The electron, which has a mass of about 0.05% that of a proton, has a charge of -1. And the positron, which is the antimatter cousin of the electron (and the same mass), has a charge of +1.
Thus, on energy and charge conservation grounds, a proton (heavy, charge +1) could decay into a positron (very light, charge +1) and a photon (very light, charge 0); however, this decay is not seen. The reason centers on the last of the relevant conserved quantities: lepton number and baryon number. Particles like the proton and neutron are baryons. They are heavy and contain three quarks. Particles like the electron are leptons. They are light and contain no quarks.
All baryons have a baryon number of B = +1, while all leptons have a lepton number of L = +1. The antimatter versions of particles have the opposite lepton or baryon number. And some particles that are neither leptons nor baryons have both a lepton and baryon number of zero.
This explains how a neutron (charge 0, mass 939.6 MeV, baryon number +1, lepton number 0) can decay into a proton (charge +1, mass 938.3 MeV, baryon number +1, lepton number 0), an electron (charge -1, mass 0.511 MeV, baryon number 0, lepton number +1), and a particle called an antimatter electron neutrino (charge 0, mass 0 MeV, baryon number 0, lepton number -1). If you check each conserved quantity, you find that it all works out.
For a proton, it is simply the lightest known baryon. Because of energy conservation, in order for it to decay into a lighter particle, there must be a lighter baryon. Since there is none, the proton is stable. For the electron, there are leptons lighter than the electron (the neutrinos), but there are no charged particles that are lighter. The lack of any combination of particles that will simultaneously obey all of the conservation laws is a barrier to electron decay.
Are protons eternal?
So, does this mean that protons and electrons are eternal? Will they last forever? This is an experimental question, not a theoretical one. Certainly, it is possible to imagine some theory governing matter that violates conservation laws and allows for protons to decay; however, scientists have built large detectors, weighing tens of thousands of tons, to search for proton decay and none has been observed. These experiments set a minimum lifetime for protons, and it is about 2 x 1034 years, or about a trillion, trillion times longer than the lifetime of the Universe. If protons decay, they exist for a very, very, long time.
Still, scientists continue to look for proton decay in experiments built for other purposes. If proton decay is ever observed, it will tell us something new and important about the laws of nature. The Deep Underground Neutrino Experiment (DUNE) is a large experiment being built to study the behavior of a subatomic particle called neutrino. While its primary purpose is neutrino studies, scientists will also exploit the detector to search for proton decay. DUNE is currently being built and is expected to begin operations toward the end of the decade.