Could getting rid of old cells turn back the clock on aging?
James Kirkland started his career in 1982 as a geriatrician, treating aging patients. But he found himself dissatisfied with what he could offer them.
“I got tired of prescribing wheelchairs, walkers and incontinence devices,” recalls Kirkland, now at the Mayo Clinic in Rochester, Minnesota. He knew that aging is considered the biggest risk factor for chronic illness, but he was frustrated by his inability to do anything about it. So Kirkland went back to school to learn the skills he’d need to tackle aging head-on, earning a PhD in biochemistry at the University of Toronto. Today, he and his colleague Tamara Tchkonia, a molecular biologist at the Mayo Clinic, are leaders in a growing movement to halt chronic disease by protecting brains and bodies from the biological fallout of aging.
If these researchers are successful, they’ll have no shortage of customers: People are living longer, and the number of Americans age 65 and older is expected to double, to 80 million, by 2040. While researchers like Kirkland don’t expect to extend lifespan, they hope to lengthen “health span,” the time that a person lives free of disease.
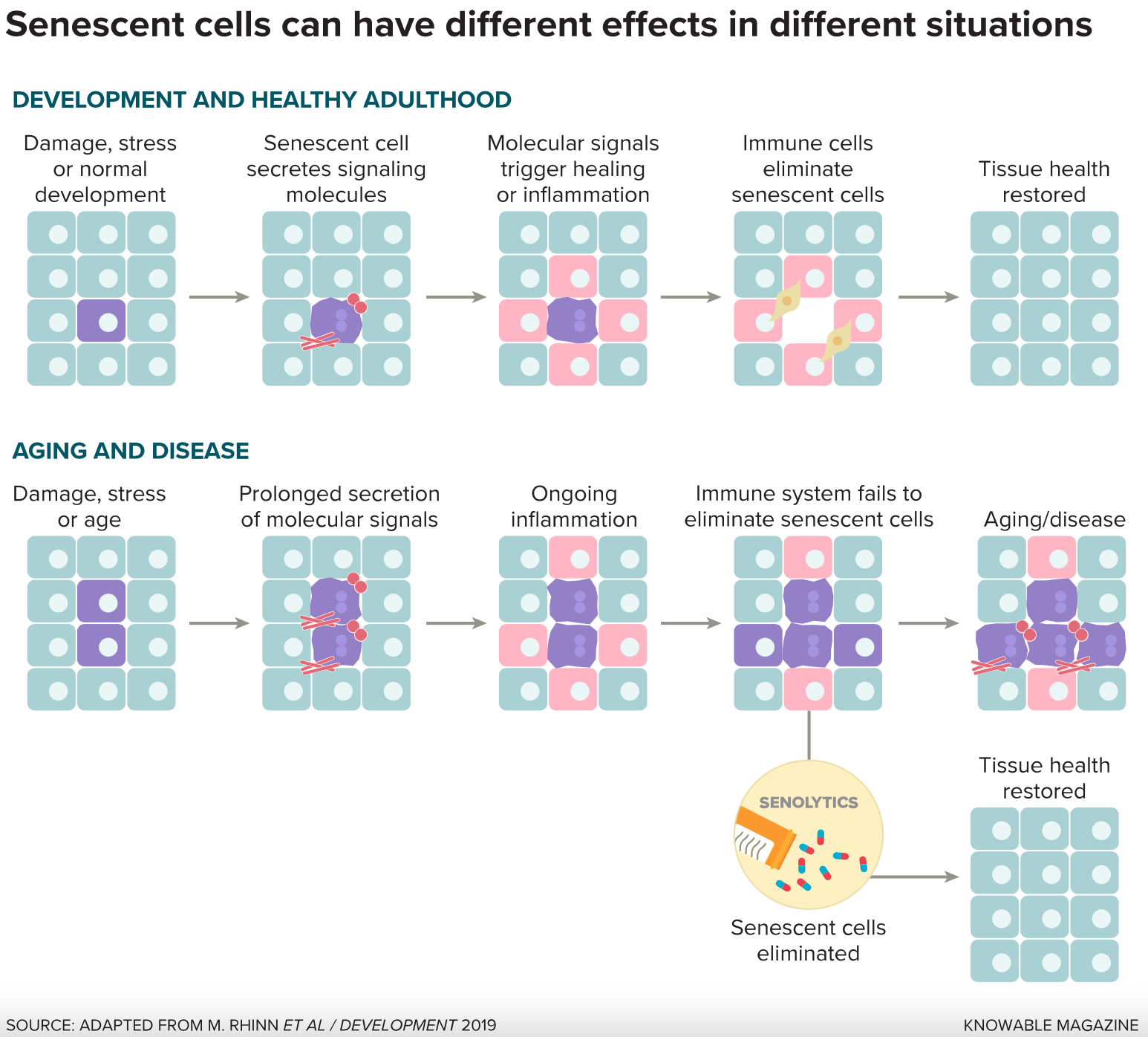
One of their targets is decrepit cells that build up in tissues as people age. These “senescent” cells have reached a point — due to damage, stress or just time — when they stop dividing, but don’t die. While senescent cells typically make up only a small fraction of the overall cell population, they accounted for up to 36 percent of cells in some organs in aging mice, one study showed. And they don’t just sit there quietly. Senescent cells can release a slew of compounds that create a toxic, inflamed environment that primes tissues for chronic illness. Senescent cells have been linked to diabetes, stroke, osteoporosis and several other conditions of aging.
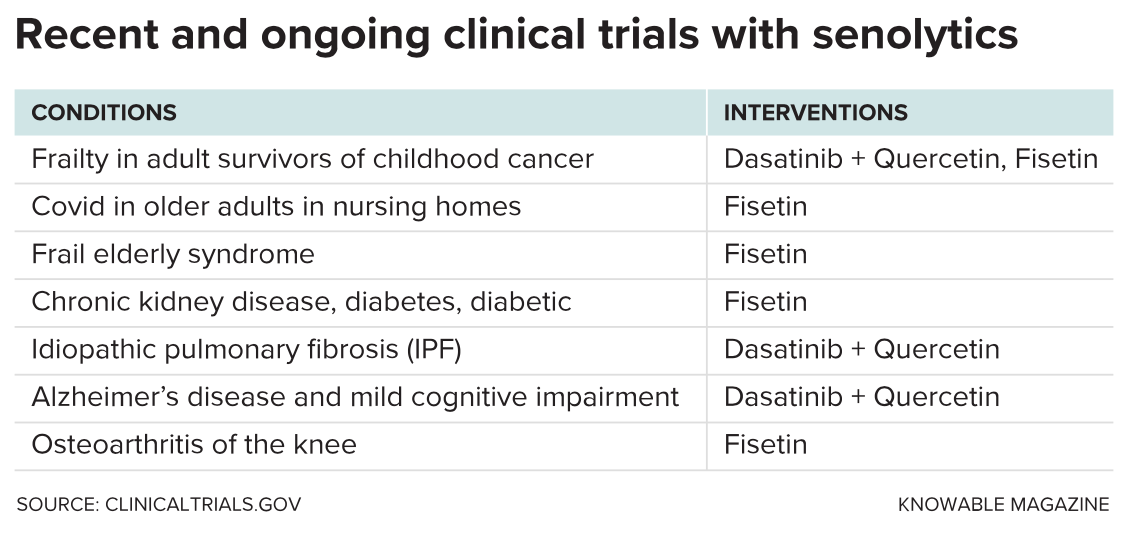
These noxious cells, along with the idea that getting rid of them could mitigate chronic illnesses and the discomforts of aging, are getting serious attention. The US National Institutes of Health is investing $125 million in a new research effort, called SenNet, that aims to identify and map senescent cells in the human body as well as in mice over the natural lifespan. And the National Institute on Aging has put up more than $3 million over four years for the Translational Geroscience Network multicenter team led by Kirkland that is running preliminary clinical trials of potential antiaging treatments. Drugs that kill senescent cells — called senolytics — are among the top candidates. Small-scale trials of these are already underway in people with conditions including Alzheimer’s, osteoarthritis and kidney disease.
“It’s an emerging and incredibly exciting, and maybe even game-changing, area,” says John Varga, chief of rheumatology at the University of Michigan Medical School in Ann Arbor, who isn’t part of the TGN.
But he and others sound a note of caution as well, and some scientists think the field’s potential has been overblown. “There’s a lot of hype,” says Varga. “I do have, I would say, a very healthy skepticism.” He warns his patients of the many unknowns and tells them that trying senolytic supplementation on their own could be dangerous.
Researchers are still untangling the biology of senescent cells, not only in aging animals but in younger ones too — even in embryos, where the aging out of certain cells is crucial for proper development. So far, evidence that destroying senescent cells helps to improve health span mostly comes from laboratory mice. Only a couple of preliminary human trials have been completed, with hints of promise but far from blockbuster results.
Even so, Kirkland and Tchkonia speculate that senolytics might eventually help not only with aging but also with conditions suffered by younger people due to injury or medical treatments such as chemotherapy. “There may be applications all over the place,” muses Kirkland.
Good cells gone bad
Biologists first noticed senescence when they began growing cells in lab dishes more than 60 years ago. After about 50 cycles of cells first growing, then dividing, the rate of cell division slows and ultimately ceases. When cells reach this state of senescence, they grow larger and start exhibiting a variety of genetic abnormalities. They also accumulate extra lysosomes, baglike organelles that destroy cellular waste. Scientists have found a handy way to identify many senescent cells by using stains that turn blue in the presence of a lysosome enzyme, called beta-galactosidase, that’s often overactive in these cells.
Scientists have also discovered hundreds of genes that senescent cells activate to shut down the cell’s replication cycle, change their biology and block natural self-destruct mechanisms. Some of these genes produce a suite of immune molecules, growth factors and other compounds. The fact that specific genes consistently turn on in senescent cells indicates there may be more to senescence than just cells running out of steam. It suggests that senescence is a cellular program that evolved for some purpose in healthy bodies. Hints at that purpose have emerged from studies of creatures far earlier in their lifespan — even before birth.
Cell biologist Bill Keyes was working on senescence in embryos back in the early 2000s. When he stained healthy mouse and chick embryos to look for beta-galactosidase, little blue spots lit up in certain tissues. He soon met up with Manuel Serrano, a cell biologist at the Institute for Research in Biomedicine in Barcelona, who’d noticed the same thing. Cells with signs of senescence turned up in the developing brain, ear and limbs, Keyes and Serrano reported in 2013.
Keyes, now at the Institute of Genetics and Molecular and Cellular Biology in Strasbourg, France, focused on mouse and chick embryonic limbs, where a thread of temporary tissue forms across the future toe-tips. Unlike most embryonic cells, the cells in this thread of tissue disappear before the animal is born. They release chemicals that help the limb develop, and once their work is done, they die. At a molecular level, they look a lot like senescent cells.
Serrano, meanwhile, looked at cells in an organ that exists only in embryos: a temporary kidney, called the mesonephros, that forms near the heart. Once the final kidneys develop, the mesonephros disappears. Here too, beta-galactosidase and other compounds linked to senescence appeared in mouse embryos.
The cells in these temporary tissues probably disappear because they are senescent. Certain compounds made by senescent cells call out to the immune system to come in and destroy the cells once their work is done. Scientists think the short-term but crucial jobs these cells perform could be the reason senescence evolved in the first place.
Other studies suggest that senescent cells may also promote health in adult animals. Judith Campisi, a cell biologist at the Buck Institute for Research on Aging in Novato, California, and others have found senescent cells in adult mice, where they participate in wound healing. Connective-tissue cells called fibroblasts fill in a wound, but if they stick around, they form abnormal scar tissue. During normal wound healing, they turn senescent, releasing compounds that both promote repair of the tissue and call immune cells to come in and destroy them.
In other words, the emergence of senescent cells in aging people isn’t necessarily a problem in and of itself. The problem seems to be that they hang around for too long. Serrano suspects this happens because the immune system in aging individuals isn’t up to the task of eliminating them all. And when senescent cells stay put, the cocktail of molecules they produce, and the ongoing immune response, can damage surrounding tissues.
Senescence can also contribute to cancer, as Campisi has described in the Annual Review of Physiology, but the relationship is multifaceted. Senescence itself is a great defense against cancer — cells that don’t divide don’t form tumors. On the other hand, the molecules senescent cells emit can create an inflamed, cancer-promoting environment. So if a senescent cell arises near a cell that’s on its way to becoming cancerous, it might alter the locale enough to push that neighbor cell over the edge. In fact, Campisi reported in 2001 that injecting mice with senescent cells made tumors grow bigger faster.
Mighty mice
If senescent cells in an aging body are bad, removing them should be good. To test this idea, Darren Baker, a molecular cell biologist at the Mayo Clinic, devised a way to kill senescent cells in mice. Baker genetically engineered mice so that when their cells turned senescent, those cells became susceptible to a certain drug. The researchers began injecting the drug twice a week once the mice turned one year old — that’s about middle age for a lab mouse.
Treated mice maintained healthier kidney, heart, muscle and fat tissue compared with untreated mice, and though they were still susceptible to cancer, tumors appeared later in life, the researchers reported in studies in 2011 and 2016. The rodents also lived, on average, five or six months longer.
These results generated plenty of interest, Baker recalls, and set senescence biology on the path toward clinical research. “That was the boom — a new era for cellular senescence,” says Viviana Perez, former program officer for the SenNet consortium at the National Institute on Aging.
Baker followed up with a study of mice that had been genetically modified to develop characteristics of Alzheimer’s. Getting rid of senescent cells staved off the buildup of toxic proteins in the brain, he reported, and seemed to help the mice to retain mental acuity, as measured by their ability to remember a new smell.
Of course, geriatricians can’t go about genetically engineering retirees, so Kirkland, Tchkonia and colleagues went hunting for senolytic drugs that would kill senescent cells while leaving their healthy neighbors untouched. They reasoned that since senescent cells appear to be resistant to a process called apoptosis, or programmed cell death, medicines that unblock that process might have senolytic properties.
Some cancer drugs do this, and the researchers included several of these in a screen of 46 compounds they tested on senescent cells grown in lab dishes. The study turned up two major winners: One was the cancer drug dasatinib, an inhibitor of several natural enzymes that appears to make it possible for the senescent cells to self-destruct. The other was quercetin, a natural antioxidant that’s responsible for the bitter flavor of apple peels and that also inhibits several cellular enzymes. Each drug worked best on senescent cells from different tissues, the scientists found, so they decided to use them both, in a combo called D+Q, in studies with mice.
In one study, Tchkonia and Kirkland gave D+Q to 20-month-old mice and found that the combination improved the rodents’ walking speed and endurance in lab tests, as well as their grip strength. And treating two-year-old mice — the equivalent of a 75- to 90-year-old human — with D+Q every other week extended their remaining lifespan by about 36 percent, compared with mice that didn’t receive senolytics, the researchers reported in 2018. Tchkonia, Kirkland and Baker all hold patents related to treating diseases by eliminating senescent cells.
To the clinic
Scientists have since discovered several other medications with senolytic effects, though D+Q remains a favorite pairing. Further studies from several research groups reported that senolytics appear to protect mice against a variety of conditions of aging, including the metabolic dysfunction associated with obesity, vascular problems associated with atherosclerosis, and bone loss akin to osteoporosis.
“That’s a big deal, collectively,” says Laura Niedernhofer, a biochemist at the University of Minnesota Medical School in Minneapolis who is a collaborator on some of these studies and a member of the TGN clinical trials collaboration. “It would be a shame not to test them in humans.”
A few small human trials have been completed. The first, published in 2019, addressed idiopathic pulmonary fibrosis, a fatal condition in which the lungs fill up with thick scar tissue that interferes with breathing. It’s most common in people 60 or older, and there’s no cure. In a small pilot study, Kirkland, Tchkonia and collaborators administered D+Q to 14 people with the condition, three times a week for three weeks. They reported notable improvement in the ability of participants to stand up from a chair and to walk for six minutes. But the study had significant caveats: In addition to its small size and short duration, there was no control group, and every participant knew they’d received D+Q. Moreover, the patients’ lung function didn’t improve, nor did their frailty or overall health.
Niedernhofer, who wasn’t involved in the trial, calls the results a “soft landing”: There seemed to be something there, but no major benefits emerged. She says she would have been more impressed with the results if the treatment had reduced the scarring in the lungs.
The TGN is now running several small trials for conditions related to aging, and other diseases too. Kirkland thinks that senescence may even be behind conditions that affect young people, such as osteoarthritis due to knee injuriesand frailty in childhood cancer survivors.
Tchkonia and Kirkland are also investigating how space radiation affects indications of senescence in the blood and urine of astronauts, in conjunction with two companies, SpaceX and Axiom Space. They hypothesize that participants in future long-term missions to Mars might have to monitor their bodies for senescence or pack senolytics to stave off accelerated cellular aging caused by extended exposure to radiation.
Kirkland is also collaborating with researchers who are investigating the use of senolytics to expand the pool of available transplant organs. Despite desperate need, about 24,000 organs from older donors are left out of the system every year because the rate of rejection is higher for these than for younger organs, says Stefan Tullius, chief of transplant surgery at Brigham and Women’s Hospital in Boston. In heart transplant experiments with mice, he reported that pretreating older donor mice with D+Q before transplant into younger recipients resulted in the donor organs working “as well or slightly better” than hearts from young donors.
“That was huge,” says Tullius. He hopes to be doing clinical trials in people within three years.
Healthy skepticism
Numerous medical companies have jumped on the anti-senescence bandwagon, notes Paul Robbins, a molecular biologist at the University of Minnesota Medical School. But results have been mixed. One front-runner, Unity Biotechnology of South San Francisco, California, dropped a top program in 2020 after its senolytic medication failed to reduce pain in patients with knee osteoarthritis.
“I think we just don’t know enough about the right drug, the right delivery, the right patient, the right biomarker,” says the University of Michigan’s Varga, who is not involved with Unity. More recently, however, the company reported progress in slowing diabetic macular edema, a form of swelling in the back of the eye due to high blood sugar.
Despite the excitement, senolytic research remains in preliminary stages. Even if the data from TGN’s initial, small trials look good, they won’t be conclusive, says network member Robbins — who nonetheless thinks positive results would be a “big deal.” Success in a small study would suggest it’s worth investing in larger studies, and in the development of drugs that are more potent or specific for senescent cells.
“I’m urging extreme caution,” says Campisi — who is herself a cofounder of Unity and holds several patents related to anti-senescence treatments. She’s optimistic about the potential for research on aging to improve health, but worries that moving senolytics quickly into human trials, as some groups are doing, could set the whole field back. That’s what happened with gene therapy in the late 1990s when an experimental treatment killed a study volunteer. “I hope they don’t kill anyone, seriously,” she says.
Side effects are an ongoing concern. For example, dasatinib (the D in D+Q) has a host of side effects ranging from nosebleeds to fainting to paralysis.
But Kirkland thinks that may not be an insurmountable problem. He notes that these side effects show up only in cancer patients taking the drug regularly for months at a time, whereas anti-senescence treatments might not need to be taken so often — once every two or three months might be enough to keep the population of senescent cells under control.
Another way to reduce the risks would be to make drugs that target senescent cells in specific tissues, Niedernhofer and Robbins note in the Annual Review of Pharmacology and Toxicology. For example, if a person has senescent cells in their heart, they could take a medicine that targets only those cells, leaving any other senescent cells in the body — which still might be doing some good — alone.
For that strategy to work, though, doctors would need better ways to map senescent cells in living people. While identifying such biomarkers is a major goal for SenNet, Campisi suspects it will be hard to find good ones. “It’s not a simple problem,” she says.
A lot of basic and clinical research must happen first, but if everything goes right, senolytics might someday be part of a personalized medicine plan: The right drugs, at the right time, could help keep aging bodies healthy and nimble. It may be a long shot, but to many researchers, the possibility of nixing walkers and wheelchairs for many patients makes it one worth taking.
This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Sign up for Knowable Magazine’s newsletter.