The neurons that make us feel hangry

Maybe it starts with a low-energy feeling, or maybe you’re getting a little cranky. You might have a headache or difficulty concentrating. Your brain is sending you a message: You’re hungry. Find food.
Studies in mice have pinpointed a cluster of cells called AgRP neurons near the underside of the brain that may create this unpleasant hungry, even “hangry,” feeling. They sit near the brain’s blood supply, giving them access to hormones arriving from the stomach and fat tissue that indicate energy levels. When energy is low, they act on a variety of other brain areas to promote feeding.
By eavesdropping on AgRP neurons in mice, scientists have begun to untangle how these cells switch on and encourage animals to seek food when they’re low on nutrients, and how they sense food landing in the gut to turn back off. Researchers have also found that the activity of AgRP neurons goes awry in mice with symptoms akin to those of anorexia, and that activating these neurons can help to restore normal eating patterns in those animals.
Understanding and manipulating AgRP neurons might lead to new treatments for both anorexia and overeating. “If we could control this hangry feeling, we might be better able to control our diets,” says Amber Alhadeff, a neuroscientist at the Monell Chemical Senses Center in Philadelphia.
To eat or not to eat
AgRP neurons appear to be key players in appetite: Deactivating them in adult mice causes the animals to stop eating — they may even die of starvation. Conversely, if researchers activate the neurons, mice hop into their food dishes and gorge themselves.
Experiments at several labs in 2015 helped to illustrate what AgRP neurons do. Researchers found that when mice hadn’t had enough to eat, AgRP neurons fired more frequently. But just the sight or smell of food — especially something yummy like peanut butter or a Hershey’s Kiss — was enough to dampen this activity, within seconds. From this, the scientists concluded that AgRP neurons cause animals to seek out food. Once food has been found, they stop firing as robustly.
One research team, led by neuroscientist Scott Sternson at the Janelia Research Campus in Ashburn, Virginia, also showed that AgRP neuron activity appears to make mice feel bad. To demonstrate this, the scientists engineered mice so that the AgRP neurons would start firing when light was shone into the brain with an optical fiber (the fiber still allowed the mice to move around freely). They placed these engineered mice in a box with two distinct areas: one colored black with a plastic grid floor, the other white with a soft, tissue paper floor. If the researchers activated AgRP neurons whenever the mice went into one of the two areas, the mice started avoiding that region.
Sternson, now at the University of California San Diego, concluded that AgRP activation felt “mildly unpleasant.” That makes sense in nature, he says: Any time a mouse leaves its nest, it’s at risk from predators, but it must overcome this fear in order to forage and eat. “These AgRP neurons are kind of the push that, in a dangerous environment, you’re going to go out and seek food to stay alive.”
Sternson’s 2015 study had shown that while the sight or smell of food quiets AgRP neurons, it’s only temporary: Activity goes right back up if the mouse can’t follow through and eat the snack. Through additional experiments, Alhadeff and colleagues discovered that what turns the AgRP neurons off more reliably is calories landing in the gut.
First, Alhadeff’s team fed mice a calorie-free treat: a gel with artificial sweetener. When mice ate the gel, AgRP neuron activity dropped, as expected — but only temporarily. As the mice learned there were no nutrients to be gained from this snack, their AgRP neurons responded less and less to each bite. Thus, as animals learn whether a treat really nourishes them, the neurons adjust the hunger dial accordingly.
Next, the team used a catheter implanted through the abdomen to deliver calories, in the form of the nutritional drink Ensure, directly to the stomach. This bypassed any sensory cues that food was coming. And it resulted in a longer dip in AgRP activity. In other words, it’s the nutrients in food that shut off AgRP neurons for an extended time after a meal, Alhadeff concluded.
Alhadeff has since begun to decode the messages that the stomach sends to the AgRP neurons, and found that it depends on the nutrient. Fat in the gut triggers a signal via the vagus nerve, which reaches from the digestive tract to the brain. The simple sugar glucose signals the brain via nerves in the spinal cord.
Her team is now investigating why these multiple paths exist. She hopes that by better understanding how AgRP neurons drive food-seeking, scientists can eventually come up with ways to help people keep off unhealthy pounds. Though scientists and dieters have been seeking such treatments for more than a century, it’s been difficult to identify easy, safe and effective treatments. The latest class of weight-loss medications, such as Wegovy, act in part on AgRP neurons but have unpleasant side effects such as nausea and diarrhea.
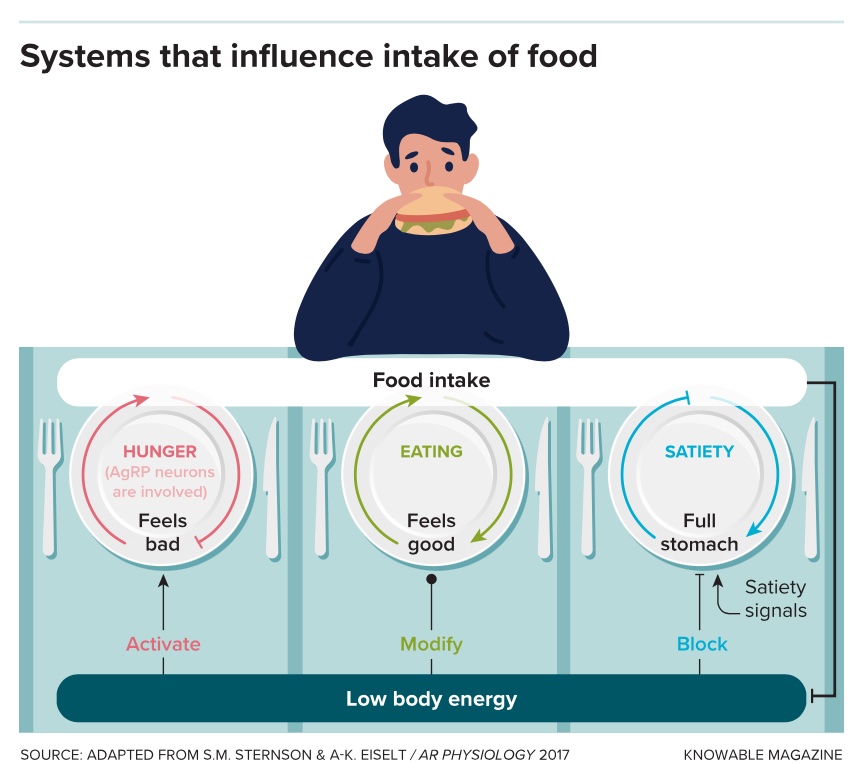
Therapies targeting AgRP neurons alone would likely fail to fully solve the weight problem, because food-seeking is only one component of appetite control, says Sternson, who reviewed the main controllers of appetite in the Annual Review of Physiology in 2017. Other brain areas that sense satiety and make high-calorie food pleasurable also play important roles, he says. That’s why, for example, you eat that slice of pumpkin pie at the end of the Thanksgiving meal, even though you’re already full of turkey and mashed potatoes.
Outflanking anorexia
The flip side of overeating is anorexia, and there, too, researchers think that investigating AgRP neurons could lead to new treatment strategies. People with anorexia avoid food, to the point of dangerous weight loss. “Eating food is actually aversive,” says Ames Sutton Hickey, a neuroscientist at Temple University in Philadelphia. There is no medication specific for anorexia; treatment may include psychotherapy, general medications such as antidepressants and, in the most severe cases, force-feeding via a tube threaded through the nose. People with anorexia are also often restless or hyperactive and may exercise excessively.
Researchers can study the condition using a mouse model of the disease known as activity-based anorexia, or ABA. When scientists limit the food available to the mice and provide them with a wheel to run on, some mice enter an anorexia-like state, eating less than they’re offered, and running on the wheel even during daylight, when mice are normally inactive. “It’s a remarkable addictive thing that happens to these animals,” says Tamas Horvath, a neuroscientist at the Yale School of Medicine. “They basically get a kick out of not eating and exercising.”
It’s not a perfect model for anorexia. Mice, presumably, face none of the social pressures to stay thin that humans do; conversely, people with anorexia usually don’t have limits on their access to food. But it’s one of the best anorexia mimics out there, says Alhadeff: “I think it’s as good as we get.”
To find out how AgRP neurons might be involved in anorexia, Sutton Hickey carefully monitored the food intake of ABA mice. She compared them to mice that were given a restricted diet, but had a locked exercise wheel and didn’t develop ABA. The ABA mice, she found, ate fewer meals than the other mice. And when they did eat, their AgRP activity didn’t decrease like it should have after they filled their tummies. Something was wrong with the way the neurons responded to hunger and food cues.
Sutton Hickey also found that she could fix the problem when she engineered ABA mice so that AgRP neurons would spring into action when researchers injected a certain chemical. These mice, when treated with the chemical, ate more meals and gained weight. “That speaks very much to the importance of these neurons,” says Horvath, who wasn’t involved in the work. “It shows that these neurons are good guys, not the bad guys.”
Sutton Hickey says the next step is to figure out why the AgRP neurons respond abnormally in ABA mice. She hopes there might be some key molecule she could target with a drug to help people with anorexia.
All in all, the work on AgRP neurons is giving scientists a much better picture of why we eat when we do — as well as new leads, perhaps, to medications that might help people change disordered eating, be it consuming too much or too little, into healthy habits.
This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Sign up for Knowable Magazine’s newsletter.