Ask Ethan: Could you have two perfectly identical snowflakes?

And when you demand ‘perfectly identical,’ just how high of a bar are you setting?
“Lives are snowflakes — unique in detail, forming patterns we have seen before, but as like one another as peas in a pod (and have you ever looked at peas in a pod? I mean, really looked at them? There’s not a chance you’d mistake one for another, after a minute’s close inspection.)” –Neil Gaiman
If you’ve ever heard someone referred to as a “special little snowflake,” the implication is that they’re beautiful and precious because of all the myriad ways in which they are unique. The old saying goes that no two snowflakes are alike, but is that really true? It’s worth looking at what the science has to say, and that’s exactly what Kara Bittner wants to know, who asks:
I know scientists say that no two snowflakes are alike, but I say “how can you definitively know that unless you can see every single snowflake that falls.” Maybe a snowflake in Russia falls [at] the same time as a snowflake in Minnesota and they’re the same.
To consider this scientifically, we have to know what goes into a snowflake, and how likely or unlikely it is that we’ll get two that are the same.
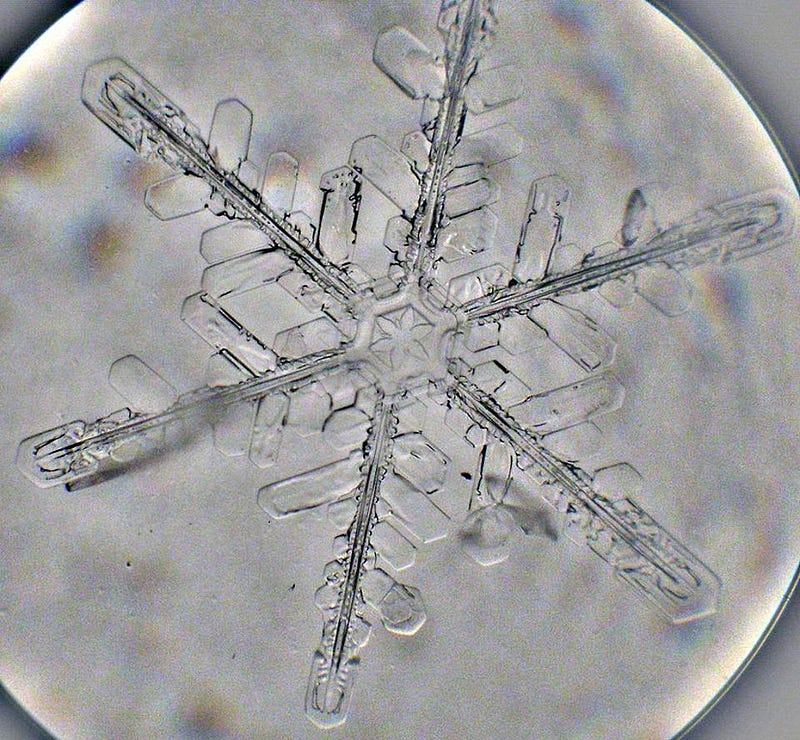
A snowflake, at its core, is just water molecules that bind together into a particular solid configuration. Most of these configurations have some sort of hexagonal symmetry; this is due to the ways that water molecules with their particular bond angles — defined by the physics of an oxygen atom, two hydrogen atoms, and the electromagnetic force — can bind together. The simplest microscopic snow crystal that can be seen through a microscope is about one-millionth of a meter across (1 µm), and might take on a very simplistic shape, like a hexagonal plate crystal. This is only approximately 10,000 atoms across, and there are a great many that look the same.

According to the Guinness book of world records, Nancy Knight, a scientist at the National Center for Atmosphere Research, serendipitously discovered two identical examples of snowflakes while studying snow crystals from a storm in Wisconsin in 1988, using a microscope. But when Guinness certifies two snowflakes as identical, they can only mean that it’s identical to the precision of the microscope; when physics demands that two things be identical, they mean identical down to the subatomic particle! That means:
- You need the same exact particles,
- In the same exact configuration,
- With the same bonds between them,
- In two entirely different macroscopic systems.
Let’s examine what it would take to get there.

A single water molecule is one oxygen atom and two hydrogen atoms bound together. When frozen water molecules bind together, each molecule gets four other water molecules bound near it: one at each of the tetrahedral vertices centered on each individual molecule. This causes water molecules to pack into a lattice shape: a hexagonal crystal lattice. But big prism-shaped “cubes” of ice, like you see when you look at a deposit of quartz, are exceedingly rare. Once you get past the smallest scales and configurations, you find that the top-and-bottom planes of this lattice are packed very closely and linked: you get “plane faces” on two of the sides. On the contrary, the remaining sides have their molecules much more exposed, and so how additional water molecules bond to them are much more arbitrary. In particular, the hexagonal corners have the weakest bonds, and that’s why there seems to be a six-fold symmetry in how hexagonal crystals grow.

The new structures themselves then grow in this same symmetric pattern, again growing hexagonal asymmetries once a certain size is reached. A large, complex snow crystals has hundreds of easily discernible features when viewed through a microscope. Hundreds of features you can see… and approximately 10¹⁹ water molecules making up your typical snowflake, according to Charles Knight at the National Center for Atmospheric Research. For every one of those features, there are literally millions of viable locations that a new branch could form at. So how many of these new, novel features could a snowflake form and still have an identical one somewhere, somewhen?
https://www.youtube.com/watch?v=GlQVkZA5j-A
Each year, worldwide, approximately 10¹⁵ (one quadrillion) cubic feet of snow fall somewhere on Earth, with each cubic foot containing approximately a few billion (10⁹) individual snowflakes. Since Earth has been around approximately 4.5 billion years, there are right around 10³⁴ snowflakes that have fallen in the history of planet Earth. Statistically, the number of individual, unique, symmetric branching features a snowflake could have and expect to have a twin at some point in Earth’s history? Only 5. Whereas real, full-grown, natural snowflakes typically have hundreds.

It’s only if you consider the smallest, earliest-stage snowflakes that you can conceivably have two identical ones. And if you’re willing to go down to a molecular level, the situation gets far worse. Normally, oxygen has 8 protons and 8 neutrons, while hydrogen has one proton and 0 neutrons. However, about 1 out of every 500 oxygen atoms has 10 neutrons instead, while 1 out of 5000 hydrogen atoms has 1 neutron instead of 0 instead. At this rate, even if you had a perfectly hexagonal snow crystal, and you made approximately 10³⁴ snow crystals over the history of planet Earth, you’d only need to reach a size of a few thousand molecules, or a snowflake just 0.01 microns across (smaller than the wavelength of visible light) to arrive at a unique structure that the planet had never seen before.

But if you’re willing to ignore atomic and molecular differences and you’re willing to forego “natural,” you’ve got a chance. Snowflake scientist Kenneth Libbrecht of Caltech has developed a technique for creating artificial “identical twin” snowflakes and photographing them with a special microscope he’s dubbed the SnowMaster 9000.
By growing them side-by-side under particular laboratory conditions, he has shown that it’s possible to create two snowflakes that are indistinguishable.
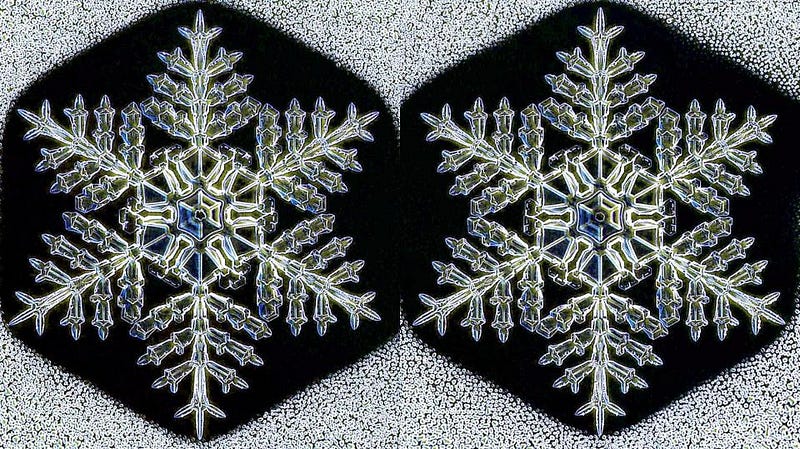
Kind of. They’re indistinguishably different to a human looking with human eyes through the microscope, but they aren’t truly identical. In fact, much like identical twins, they have many differences: they have different molecular bonding sites, they have slightly different branching properties, and the larger they get, the more pronounced these differences become. That’s why these snowflakes are kept small and the microscope is so powerful: they’re more identical when they’re less complex.

Nevertheless, many multiple snowflakes are alike, as in they’re very much like each other. But if you’re looking for truly identical, at a structural, molecular or atomic level, nature will never get you there. The number of possibilities is not just too great for the history of Earth, but for the history of the entire Universe. If you wanted to know how many Earths you’d need to have a chance of two identical snowflakes in the 13.8 billion year history of the Universe, the answer is somewhere around 10¹⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰⁰. Considering there are only about 10⁸⁰ atoms in the entire observable Universe, it’s pretty unlikely. To the best that we can say, snowflakes really are unique.
Submit your Ask Ethan questions to startswithabang at gmail dot com.
This post first appeared at Forbes, and is brought to you ad-free by our Patreon supporters. Comment on our forum, & buy our first book: Beyond The Galaxy!