Why nuclear reactions on an exoplanet won’t imply alien life

- In order to make a uranium-based nuclear reactor, the naturally occurring amount of U-235 is too low at present; we have to enrich what we find to have sufficiently large U-235/U-238 ratios.
- But 1.7 billion years ago, which is more than two full half-lives of U-235, there was a much greater abundance: enough to trigger a self-sustaining nuclear reaction under the right conditions.
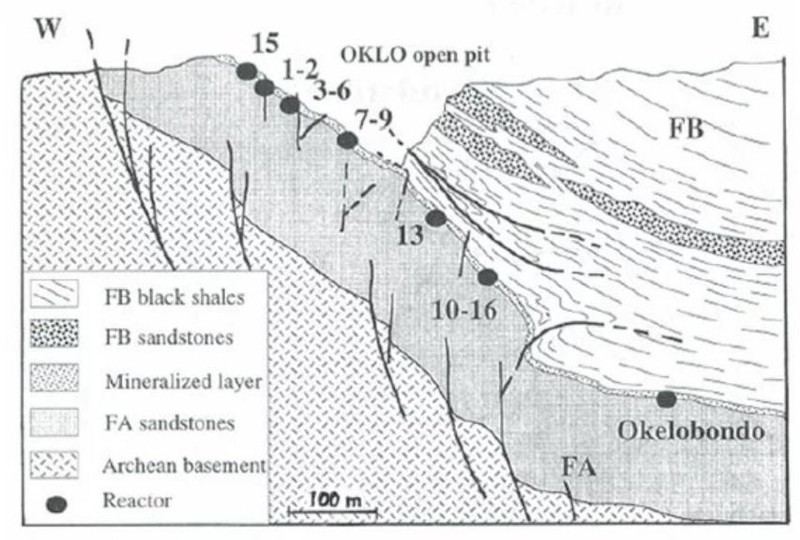
- Those conditions existed, naturally, 1.7 billion years ago in the Oklo mines of Gabon, West Africa. 17 natural sites possessing ancient nuclear reactions have now been found: evidence of Earth’s first nuclear reactor.
If you were hunting for alien intelligence, looking for a surefire signature from across the Universe of their activity, you’d have a few options.
- You could look for an intelligent radio broadcast, like the type humans began emitting in the 20th century.
- You could look for examples of planet-wide modifications, like human civilization displays when you view Earth at a high-enough resolution.
- You could look for artificial illumination at night, like our cities, towns, and fisheries display, visible from space.
And there are many other options: you can look for a unique chemical “fingerprint” of biological processes in an exoplanet’s atmosphere by performing spectroscopy on the planet’s atmosphere, or rapid long-term evolution of certain chemical species. After all, there’s a tremendous amount you can learn even from a single pixel if you can directly image an Earth-sized exoplanet.
But there are technological achievements that we’ve realized here on Earth as human civilization has advanced, and you might think to look for signatures of precisely those same achievements, such as the particle creation of antineutrinos in a nuclear reactor. After all, that’s how humanity first detected neutrinos (or antineutrinos) on Earth: back in 1956, when Reines and Cowan did it. But if we pursued that last option, we might fool ourselves into thinking there are intelligent aliens when, in fact, there are none. After all, Earth once possessed a natural nuclear reactor 1.7 billion years ago: long before humans ever existed.

In order to create a nuclear reactor today, the first ingredient we require is reactor-grade fuel. Uranium, for example, is created in stellar cataclysms, with kilonova events — or colliding and merging neutron stars that don’t immediately form a black hole — doing the majority of creation for this element and its isotopes. Here on Earth, uranium comes in two different naturally-occurring isotopes:
- U-238 (with 146 neutrons),
- and U-235 (with 143 neutrons).
Changing the number of neutrons doesn’t change your element type, but does change how stable your element is. For U-235 and U-238, they both decay via a radioactive chain reaction, but U-238 lives about six times as long, on average.
By the time you get to the present day, billions of years after the creation of the Earth (and the uranium within it), both of these isotopes of uranium have partially decayed, but more U-235 has decayed compared with U-238. U-235 makes up only about 0.72% of all naturally-occurring uranium, meaning it has to be enriched to at least about 3% levels in order to get a sustaining fission reaction, or a special setup (involving heavy water mediators) is required. But 1.7 billion years ago was more than two full half-lives ago for U-235. Back then, in ancient Earth, U-235 was about 3.7% of all uranium: enough for a reaction to occur.
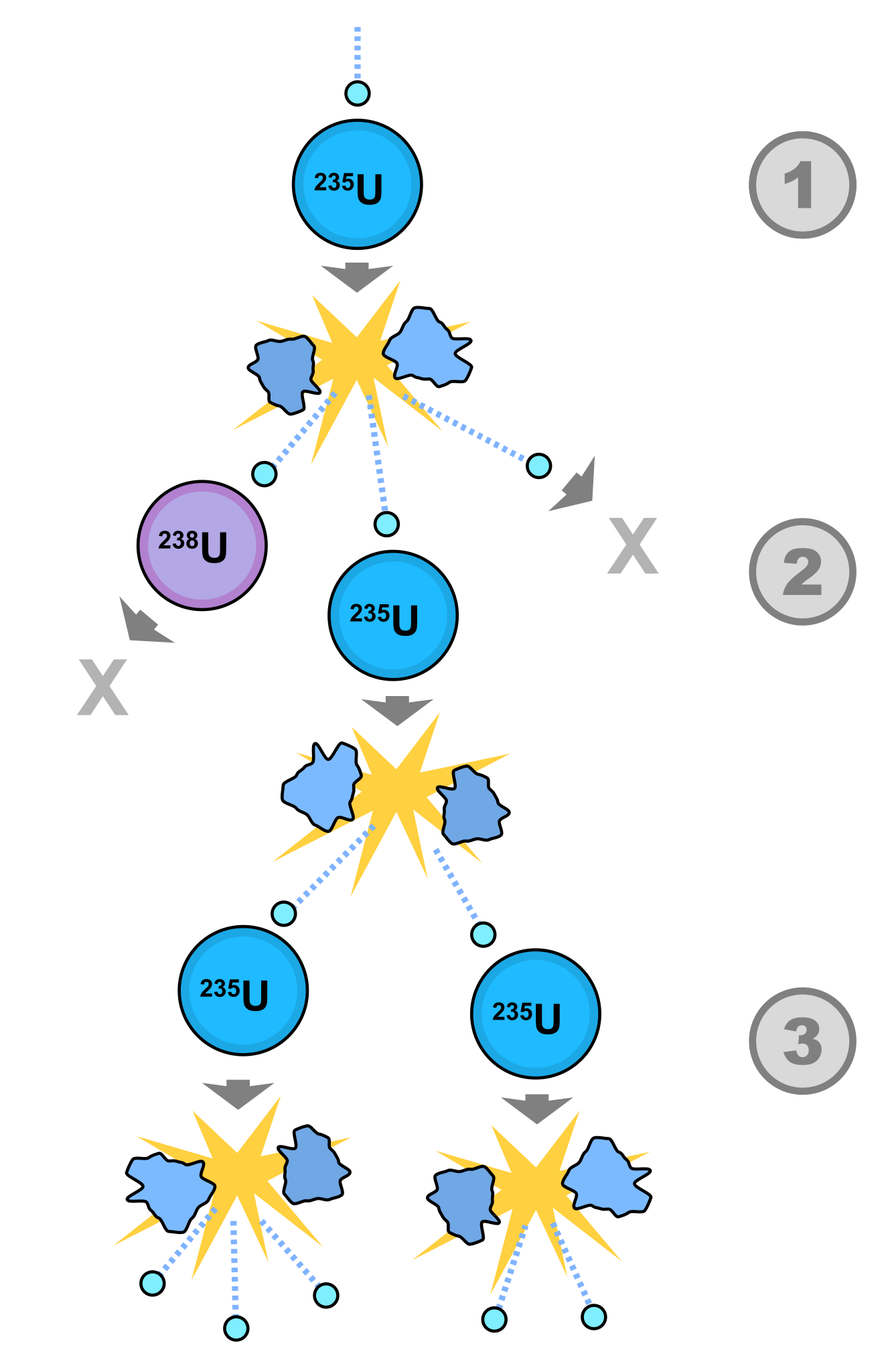
In between different layers of sandstone, before you reach the granite bedrock making up most of Earth’s crust, you often find veins of mineral deposits: sections of the Earth that are especially rich in a particular element. Sometimes these are extremely lucrative from a miner’s perspective, like when we find gold, platinum, or palladium veins underground. However, at other times, we find even rarer materials in there, such as uranium. In modern reactors, enriched uranium produces several neutrons every time a U-235 nucleus gets struck by a neutron, and since neutron emission is also a process that occurs naturally and spontaneously, a nuclear chain reaction can be triggered at almost any time.
Imagine we have an enriched vein of uranium, rich in U-235 (at the 3% or greater level of enrichment), that’s below ground somewhere within the Earth, but close to the surface. Now imagine that vein is in contact with subterranean water: also a common occurrence. In the presence of water, a U-235 nucleus gets struck by a neutron, causing it to emit more neutrons. Water then acts like a neutron moderator, and a fraction of those newly created neutrons will strike another U-235 nucleus, causing a fission reaction and leading to the creation of even more neutrons. As each U-235 nucleus splits apart, it produces lighter daughter nuclei, releases energy, and also produces three additional neutrons. If the conditions are right, the reaction will trigger additional fission events, leading to a self-sustaining reactor.
Two factors came together, 1.7 billion years ago, to create a natural nuclear reactor. The first is that, above the bedrock layer of granite, groundwater flows freely, and it’s only a matter of geology and time before water flows into the uranium-rich regions. Surround your uranium atoms with water molecules, and that’s a solid start. This is easy to imagine occurring naturally:
- the pre-solar nebula came heavily enriched with both U-235 and U-238 being abundant,
- then Earth formed, where veins rich in both isotopes of uranium appear at or near the surface,
- and then water, both fresh and salt water, can come into contact with those uranium veins, persisting in that contact for geologically long periods of time.
That’s a healthy start. But to get your reactor working well, in a self-sustaining fashion, you need an extra, second component: you want the uranium atoms to actually become dissolved in the water. In order for uranium to be soluble in water, oxygen must be present. Fortunately, aerobic, oxygen-using bacteria evolved in the aftermath of the first mass extinction in Earth’s recorded history: the great oxygenation event. With oxygen in the groundwater, dissolved uranium would be possible whenever water floods the mineral veins, and could have even created particularly uranium-rich material.

When you have a uranium fission reaction, a number of important signatures wind up being produced.
- Five isotopes of the element xenon are produced as reaction products.
- The remaining U-235/U-238 ratio should be reduced, since only U-235 is fissile.
- U-235, when split apart, produces large amounts of neodymium (Nd) with a specific weight: Nd-143. Normally, the ratio of Nd-143 to the other isotopes is about 11–12%; seeing an enhancement indicates uranium fission.
- Same deal for ruthenium with a weight of 99 (Ru-99). Naturally occurring with about 12.7% abundance, fission can increase that to about 27–30%.
- And, of course, there is a particular signature of antineutrinos (and particularly electron antineutrinos) that are produced as a by-product of these fission reactions.
In 1972, the French physicist Francis Perrin discovered a total of 17 sites spread across three ore deposits at the Oklo mines in Gabon, West Africa, that contained remnant enrichments that showed the first four of these signatures; only the fifth signature leaves no long-term trace, as you have to be there to detect it at the moment those antineutrinos are produced.

The Oklo fission reactors are the only known examples of a natural nuclear reactor here on Earth, but the mechanism by which they occurred leads us to believe that these could have occurred in many locations on our planet in the past: wherever those favorable combinations of conditions persisted. In theory, those same (or analogous) conditions could occur elsewhere in the Universe as well, perhaps even ubiquitously if the conditions that existed on Earth billions of years ago exist on many other planets in the galaxy at present.
Whenever groundwater inundates a uranium-rich mineral deposit, and enough oxygen is present to cause the uranium to dissolve within that water, a series of fission reactions, where U-235 nuclei split apart, can occur. The groundwater acts as a neutron moderator, allowing (on average) more than 1 out of 3 neutrons to collide with a U-235 nucleus, continuing the chain reaction so long as the density of uranium in that solution is great enough. However, the reaction will go on for only a short amount of time, as the groundwater that moderates the neutrons will ultimately boil away due to the heat produced by these fission reactions, which halts the reaction altogether. Over time, however, without fission occurring, the reactor naturally cools down, allowing groundwater back in, and a new set of reactions to resume.

By examining the concentrations of xenon isotopes that become trapped in the mineral formations surrounding the uranium ore deposits, humanity, like an outstanding detective, has been able to calculate the specific timeline of the Oklo natural nuclear reactor.
- For approximately 30 minutes, the reactor would go critical, with fission proceeding until the water boils away.
- Over the next ~150 minutes, there would be a cooldown period, after which water would flood the mineral ore again and fission would restart.
- This three hour cycle would repeat itself, over and over again, for hundreds of thousands of years.
- However, with each set of cyclical reactions, the ratio of U-235 compared to U-238 would decrease, as the U-235 gets split apart but the U-238 does not.
- The repeating cycle, therefore, will persist for a time, but will come to an end when the ever-decreasing amount of U-235 reaches a low-enough level: below that critical ~3% amount.
- After hundreds of thousands of years, a chain reaction will no longer be sustainable, and every stray neutron that strikes a U-235 nucleus will peter out instead of continuing the cycle.
At that point, all that both U-235 and U-238 will do is radioactively decay away, as no self-sustaining nuclear reactions will take place any longer.

Looking at the Oklo sites today, we find natural U-235 abundances that are depleted from their normal ratios by a small but very significant amount: 0.44%-to-0.60%. Although the natural abundance of the U-235 that’s normally found is incredibly low, at 0.720% U-235, compared to 99.28% U-238 (looking at the uranium alone), the Oklo samples differ from those ratios. In fact, across all of the known reactor sites that affected the mineral deposits found there, they only display U-235 abundances that range from 0.7157% up to 0.7168%: all well-below the normal value of 0.720% by substantial, statistically significant amounts.
Nuclear fission, in some form or another, is the only naturally-occurring explanation for this discrepancy. This discrepancy isn’t found anywhere else; only under the fortuitous conditions that existed within the Oklo reactor some ~1.7 billion years ago. Combined with the elemental abundances of the daughter species that are found in those same deposits — including the xenon, the neodymium, and the ruthenium evidence — the conclusion that this was a natural, geologically-created nuclear reactor is all but inescapable. There is no known alternative hypothesis that can also produce these signatures.

Interestingly enough, there are a number of scientific findings we can conclude from looking at the nuclear reactions that occurred here.
- We can determine the timescales of the on/off cycles by looking at the various xenon deposits.
- The sizes of the uranium veins and the amount that they’ve migrated (along with the other materials affected by the reactor) over the past 1.7 billion years can give us a useful, natural analogue for how to store and dispose of nuclear waste.
- The isotope ratios found at the Oklo sites allow us to test the rate of various nuclear reactions, and determine if they (or the fundamental constants driving them) have changed over time.
Based on this evidence, we can determine that the rates of nuclear reactions, and therefore the values of the constants that determine them, were the same 1.7 billion years ago as they are today.
And finally, and perhaps most importantly for understanding our planet’s natural history, we can use the ratios of the various elements to determine both Earth’s age as well as its composition at the moment of its creation. The lead-isotope and uranium-isotope levels teach us that 5.4 tonnes of fission products were produced, over a grand total of a ~2 million year timespan some 1.7 billion years ago, on an Earth that’s 4.5 billion years old today.

When neutron stars merge, plus possibly when other stellar cataclysms (such as supernovae or exploding white dwarfs) occur, both U-235 and U-238 are produced. From examining supernovae, we know we actually create more U-235 than U-238 in about a 60/40 ratio in those events, and similar ratios are expected from kilonova events. If Earth’s uranium were all created from a single supernova — a first-order approximation but not the worst approximation we can make — that supernova would have occurred about 6 billion years before the formation of Earth.
On any world, as long as a rich vein of near-surface uranium ore exists with greater than 3/97 ratio of U-235 to U-238, mediated by water, a spontaneous and natural nuclear reaction can occur. These conditions could arise at any time, and as long as few enough half-lives have passed relative to the decay time of U-235, the discovery of “reactor antineutrinos” from another world might indicate a natural nuclear reaction just as easily as it could indicate the presence of an intelligent, technologically advanced civilization creating their own nuclear reactions. The antineutrino signature can even persist for millions of years; detecting a sustained versus a transient antineutrino source will still not be a smoking gun for intelligent aliens.
In one serendipitous location on Earth, in more than a dozen instances, we have overwhelming evidence for a history of nuclear fission. In the hunt for extraterrestrial intelligence, we must take extreme care to not let a natural signal confuse us; we’ll need more unambiguous evidence than detecting nuclear fission reactions in a reactor-type environment can provide.
A version of this article was first published in September of 2022. It was updated in April of 2025.