Fireworks are only possible because of quantum physics

- Although they’re remarkable for a number of reasons, launching high into the air and detonating to produce spectacular, colored light patterns, there’s more than just combustion occurring when it comes to fireworks.
- The explosions that occur, as well as their unique and vibrant colors, are driven by quantum transitions that occur all the way down at the level of atoms.
- This 4th of July, or any time when fireworks are set off, you should appreciate just how intricately nature needs to work in order to produce these luminous displays that entertain us so thoroughly.
This Monday, July 4, 2022, is remarkable for a number of reasons. It happens to be aphelion: the day where the Earth is at its most distant from the Sun as it revolves through the Solar System in its elliptical orbit. It’s the 246th anniversary of when the United States officially declared independence from, and war on, Britain. And it marks the annual date where the wealthiest nation in the world sets off more explosives — in the form of fireworks — than any other.
Whether you’re an amateur hobbyist, a professional installer, or simply a spectator, fireworks shows are driven by the same laws of physics that govern all of nature. Individual fireworks all contain the same four component stages: launch, fuse, burst charges, and stars. Without quantum physics, not a single one of them would be possible. Here’s the science behind how every component of these spectacular shows works.
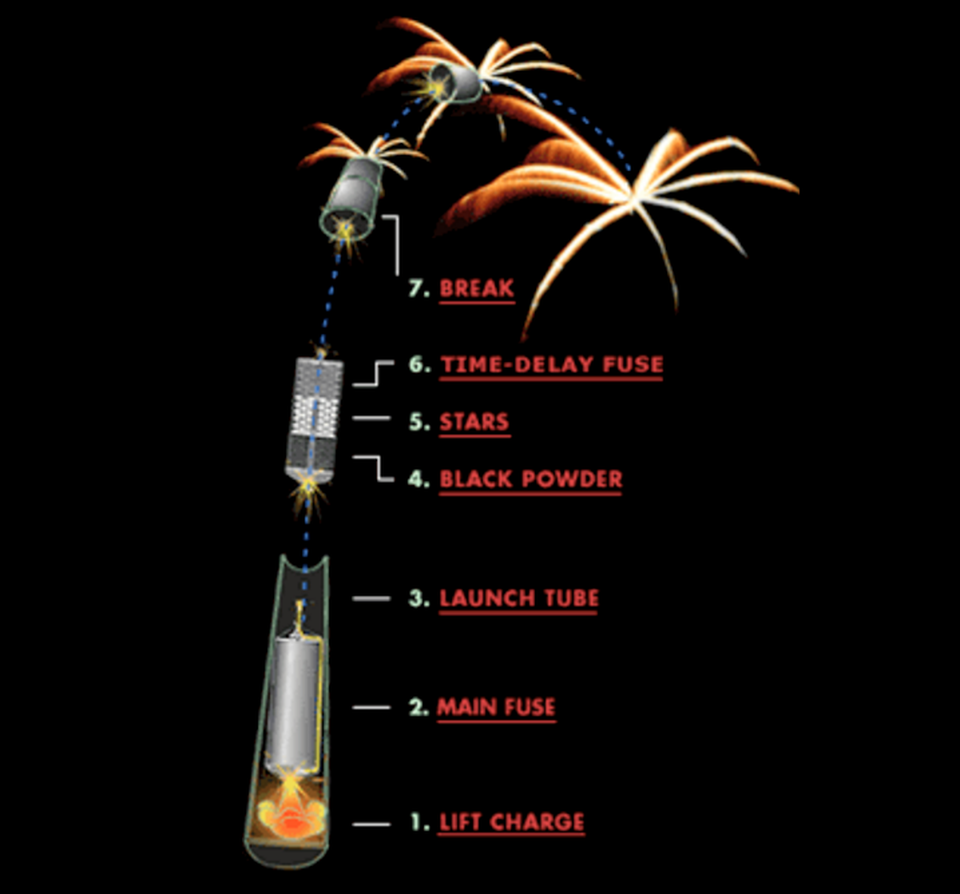
The start of any firework is the launch aspect: the initial explosion that causes the lift. Ever since fireworks were first invented more than a millennium ago, the same three simple ingredients have been at the heart of them: sulfur, charcoal, and a source of potassium nitrate. Sulfur is a yellow solid that occurs naturally in volcanically active locations, while potassium nitrate is abundant in natural sources like bird droppings or bat guano.
Charcoal, on the other hand, isn’t the briquettes we commonly use for grilling, but the carbon residue left over from charring (or pyrolyzing) organic matter, such as wood. Once all the water has been removed from the charcoal, all three ingredients can be mixed together with a mortar and pestle. The fine, black powder that emerges is gunpowder, already oxygen-rich from the potassium nitrate.

With all those ingredients mixed together, there’s a lot of stored energy in the molecular bonds holding the different components together. But there’s a more stable configuration that these atoms and molecules could be rearranged into. The raw ingredients — potassium nitrate, carbon, and sulfur — will combust (in the presence of high-enough temperatures) to form solids such as potassium carbonate, potassium sulfate, and potassium sulfide, along gases such as carbon dioxide, nitrogen, and carbon monoxide.
All it takes to reach these high temperatures is a small heat source, like a match. The reaction is a quick-burning deflagration, rather than an explosion, which is incredibly useful in a propulsion device. The rearrangement of these atoms (and the fact that the fuel contains its own oxygen) allows the nuclei and electrons to rearrange their configuration, releasing energy and sustaining the reaction. Without the quantum physics of these rearranged bonds, there would be no way to release this stored energy.

When that first energy release occurs, conventionally known as the lift charge, it has two important effects.
- The lift charge imparts an impulse, causing an acceleration, to the remainder of the firework, which includes the other three components. Because the firework is encased within a launch tube, the acceleration is always in the desired direction: upward.
- The lift charge, during the process of combustion, ignites the main fuse, which will cause the firework to detonate when it reaches the black powder inside.
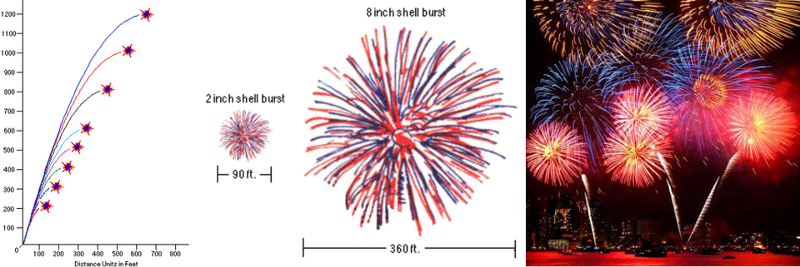
The upward acceleration needs to give your firework the right upward velocity to get it to a safe height for explosion, and the fuse needs to be timed appropriately to detonate at the peak launch height. A small fireworks show might have shells as small as 2 inches (5 cm) in diameter, which require a height of 200 feet (60 m), while the largest shows (like the one by the Statue of Liberty in New York) have shells as large as 3 feet (90 cm) in diameter, requiring altitudes exceeding 1000 feet (300 m).
The fuse, on the other hand, is the second stage and will be lit by the ignition stage of the launch. Most fuses rely on a similar black powder reaction to the one used in a lift charge, except the burning black powder core is surrounded by wrapped textile coated with either wax or lacquer. The inner core functions via the same quantum rearrangement of atoms and electron bonds as any black powder reaction, but the remaining fuse components serve a different purpose: to delay ignition.
The textile material is typically made of multiple woven and coated strings. The coatings make the device water resistant, so they can work regardless of weather. The woven strings control the rate of burning, dependent on what they’re made out of, the number and diameter of each woven string, and the diameter of the powder core. Slow-burning fuses might take 30 seconds to burn a single foot, while fast-burning fuses can burn hundreds of feet in a single second.
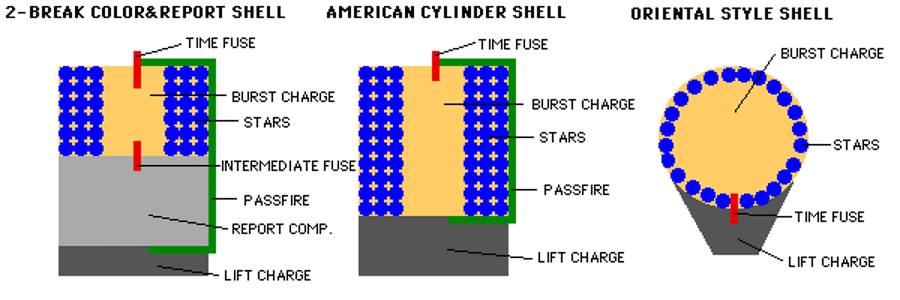
The third stage, then, is the burst charge stage, which controls the size and spatial distribution of the stars inside. In general the higher you launch your fireworks and the larger-diameter your shells are, the larger your burst charge will need to be to propel the insides of the shell outward. In general, the interior of the firework will have a fuse connected to the burst charge, which is surrounded by the color-producing stars.
The burst charge can be as simple as another collection of black powder, such as gunpowder. But it could be far more complex, such as the much louder and more impressive flash powder, or a multi-stage explosive that sends stars in multiple directions. By utilizing different chemical compounds that offer different quantum rearrangements of their bonds, you can tune your energy release, the size of the burst, and the distribution and ignition times of the stars.

But the most interesting part is that final stage: where the stars ignite. The burst is what takes the interior temperatures to sufficient levels to create the light and color that we associate with these spectacular shows. The coarse explanation is that you can take different chemical compounds, place them inside the stars, and when they reach a sufficient temperature, they emit light of different colors.
This explanation, though, glosses over the most important component: the mechanism of how these colors are emitted. When you apply enough energy to an atom or molecule, you can excite or even ionize the electrons that conventionally keep it electrically neutral. When those excited electrons then naturally cascade downward in the atom, molecule or ion, they emit photons, producing emission lines of a characteristic frequency. If they fall in the visible portion of the spectrum, the human eye is even capable of seeing them.
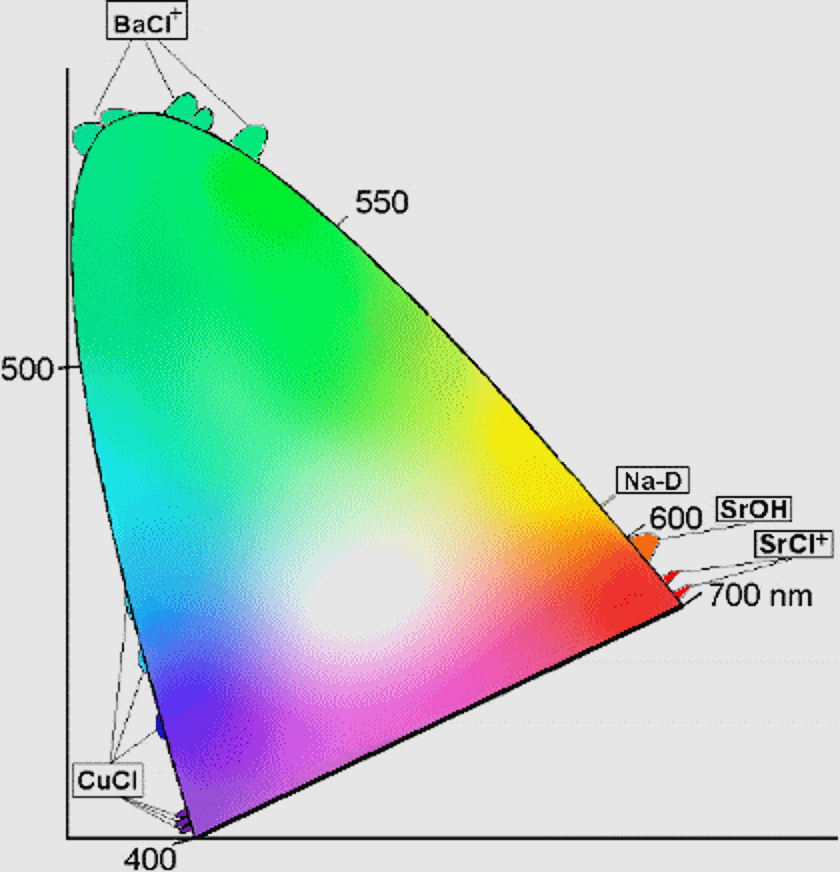
What determines which emission lines an element or compound possesses? It’s simply the quantum mechanics of the spacing between the different energy levels inherent to the substance itself. For example, heated sodium emits a characteristic yellow glow, as it has two very narrow emission lines at 588 and 589 nanometers. You’re likely familiar with these if you live in a city, as most of those yellow-colored street lamps you see are powered by elemental sodium.
As applied to fireworks, there are a great variety of elements and compounds that can be utilized to emit a wide variety of colors. Different compounds of Barium, Sodium, Copper and Strontium can produce colors covering a huge range of the visible spectrum, and the different compounds inserted in the fireworks’ stars are responsible for everything we see. In fact, the full spectrum of colors can be achieved with just a handful of conventional compounds.
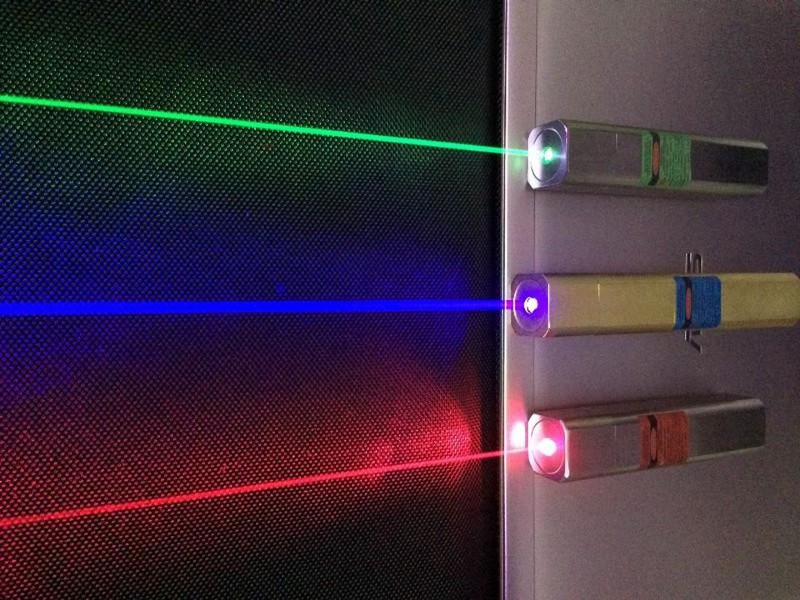
What’s perhaps the most impressive about all of this is that the color we see with the human eye is not necessarily the same as the color emitted by the fireworks themselves. For example, if you were to analyze the light emitted by a violet laser, you’d find that the photons emerging from it were of a specific wavelength that corresponded to the violet part of the spectrum.
The quantum transitions that power a laser always result in photons of exactly the same wavelength, and our eyes see them precisely as they are, with the multiple types of cones we possess responding to that signal in such a way that our brain responds to construct a signal that’s commensurate with the light possessing a violet color.
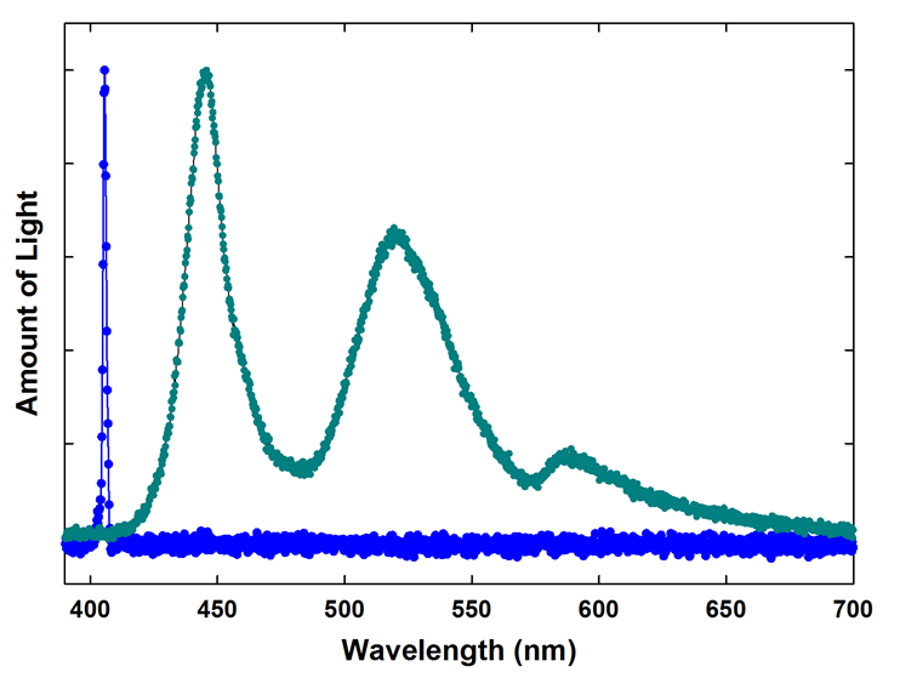
But if you look at that same color that appears as “violet” not from a monochromatic source like a laser, but from your phone or computer screen, you’ll find that there are no intrinsically violet photons striking your eyes at all! Instead, as Chad Orzel has noted in the past,
“Our eyes construct what we perceive as color from the response of three types of cells in our retina, each sensitive to light of a particular range of colors. One is most sensitive to blue-ish light (short wavelength), one is most sensitive to red light (long wavelength), and the third to a sort of yellow-green. Based on how strongly each of these cells responds to incoming light, our brains construct our perception of color.“
In other words, the key to producing the fireworks display you want isn’t necessarily to create light of a specific color that corresponds to a specific wavelength, but rather to create light that excites the right molecules in our body to cause our brain to perceive a particular color.
Fireworks might appear to be relatively simple explosive devices. Pack a charge into the bottom of a tube to lift the fireworks to the desired height, ignite a fuse of the proper length to reach the burst charge at the peak of its trajectory, explode the burst charge to distribute the stars at a high temperature, and then watch and listen to the show as the sound, light, and color washes over you.
Yet if we look a little deeper, we can understand how quantum physics underlies every single one of these reactions. Add a little bit extra — such as propulsion or fuel inside each star — and your colored lights can spin, rise, or thrust in a random direction. Make sure you enjoy your fourth of July safely, but also armed with the knowledge that empowers you to understand how the most spectacular human-made light show of the year truly works!