How does helium get underground in the first place?

If it’s such a light element, why are Earth’s stores of it buried beneath the surface?
“I have this one little saying, when things get too heavy just call me helium, the lightest known gas to man.” –Jimi Hendrix
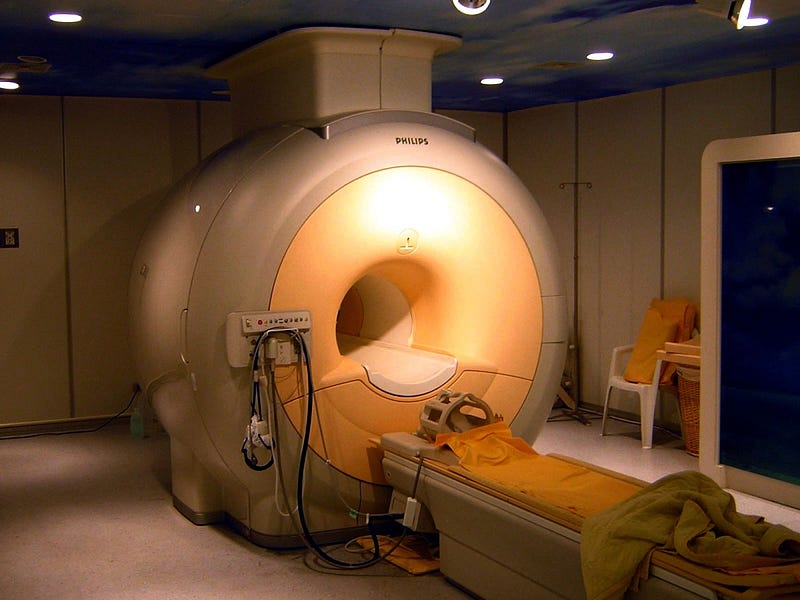
In a huge announcement this week, scientists gathered together from a number of different universities and research facilities to hail the discovery of a giant underground deposit of helium. In Tanzania, along the eastern coast of Africa, more than 50 billion cubic feet of helium was just discovered. This discovery — and the technique that enabled it — provides relief for a dwindling global reserve that is just as essential to particle accelerators and MRI machines as it is frivolously wasted on balloons and birthday parties.
Helium may be the second most abundant element in the Universe, but it’s quite a rarity on Earth. The second lightest element in the periodic table, it’s named for Helios, the ancient greek sun god, because it was discovered on the Sun, spectroscopically, before it was ever found on Earth. It wasn’t until 1882, where that same unique spectral line was seen in the lava flowing from an eruption of Mount Vesuvius CKSNY +%. It was isolated a few years later by chemically treating igneous rocks, which separated the noble gases from the atoms they were bound together with. Perhaps not ironically, the new technique the scientists used to discover the helium in Tanzania made use of this exact knowledge and set of volcanic conditions.

But helium is too light to exist on Earth for long. Once it reaches the surface in its gaseous phase — once it comes out of the rock and makes it into the atmosphere — it’s only a matter of time before it gets kicked out into interplanetary space. Helium is lighter than all the other gases in Earth’s atmosphere, and over time it rises to the very top of the exosphere: the border between Earth’s most tenuous atoms and the vacuum of space itself. At these great heights, a strong kick from either sunlight or a solar wind particle is enough to propel a helium atom past its escape velocity, and off of Earth forever. Any large amounts of helium that Earth was formed with was ejected from our planet long ago, where the current helium fraction of our atmosphere is a meagre 0.00052%.

The way you form helium on Earth, ironically, is deep inside the planet, where the heaviest elements reside. While most of what composes Earth is stable — elements like iron, nickel, silicon, oxygen, sulphur, lead and more — there are a few notable exceptions. Elements like radium, thorium and uranium, while they might compose less than 1% of the Earth, have Einstein’s greatest secret encoded into their nuclei: they’re unstable, they radioactively decay, and when they do, they convert a tiny fraction of their mass into energy via E = mc2.
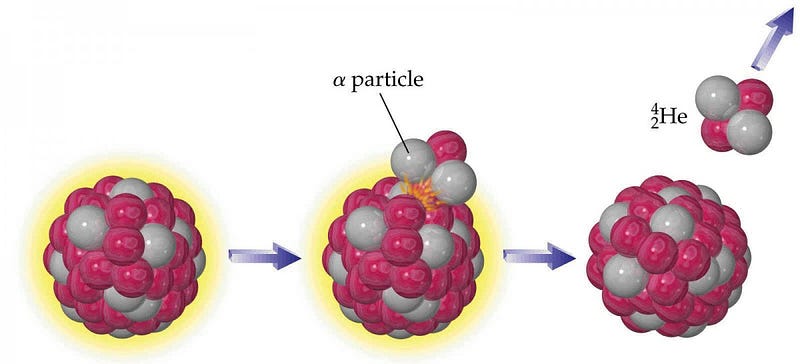
The way these elements decay is through a process known as α-decay, where a heavy nucleus emits two protons and two neutrons bound together, often participating in a decay chain where many α-decays happen in a row. The interesting thing is that configuration (an α-particle) of two protons and two neutrons, bound together, is also a helium nucleus! If you take a look at the planet as a whole, approximately 50% of the heat generated by the Earth itself comes from gravitational contraction, while the other 50% comes from radioactive decays. Deep within the Earth, the decay of these heavy elements makes our planet itself a very slow helium factory.
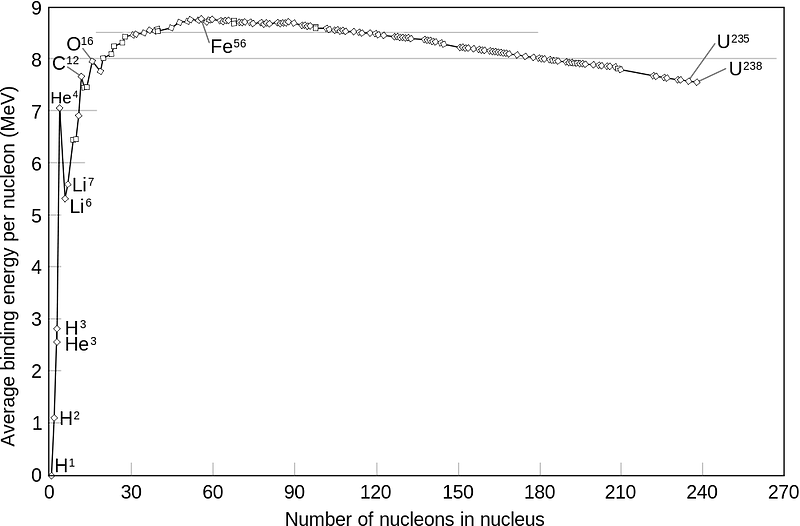
It takes hundreds of millions of years to produce any substantial amount of helium, and once we extract it, it will take that long again for these stores to replenish themselves. Helium is rare and precious in the sense that this world only gives us a one-time shot of easily harvestable, accessible, abundant stores of it. Once we waste it, it’s gone forever, making every reachable subterranean store of helium that much more precious.
This post first appeared at Forbes, and is brought to you ad-free by our Patreon supporters. Comment on our forum, & buy our first book: Beyond The Galaxy!