The 3 most surprising elements
Every element found on Earth was made in either the Big Bang or the cores of stars… except these three.
“We cannot conceive of matter being formed of nothing, since things require a seed to start from… Therefore there is not anything which returns to nothing, but all things return dissolved into their elements.” -Lucretius, De Rerum Natura
You might look around at the world and marvel at the huge diversity of things that exist in our world, both naturally and by the hands of humanity.

Yet despite the incredible complexity of things that the Universe can create, everything is made up — at a fundamental level — of relatively simple building blocks. It’s just that the way they come together is so intricate, complex and varied, that the possible combinations can produce a seemingly limitless set of outcomes.
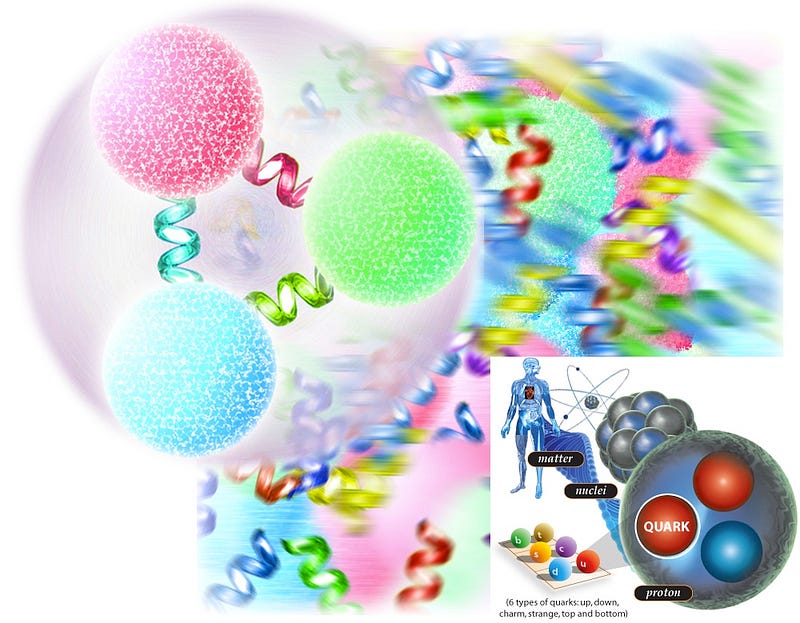
On the smallest scales, matter is mostly made up of quarks-and-gluons, which account for some 99.96% of the mass of all the things we interact with here on our world. Quarks and gluons can’t exist freely, however. We only find them bound together in two forms here on Earth: protons and neutrons.
And although individual, free neutrons are unstable, we find protons and neutrons bound together into a large number of stable combinations, forming the huge diversity of atomic nuclei we’re familiar with. When you add enough electrons to each of these nuclei, you wind up with neutral atoms.
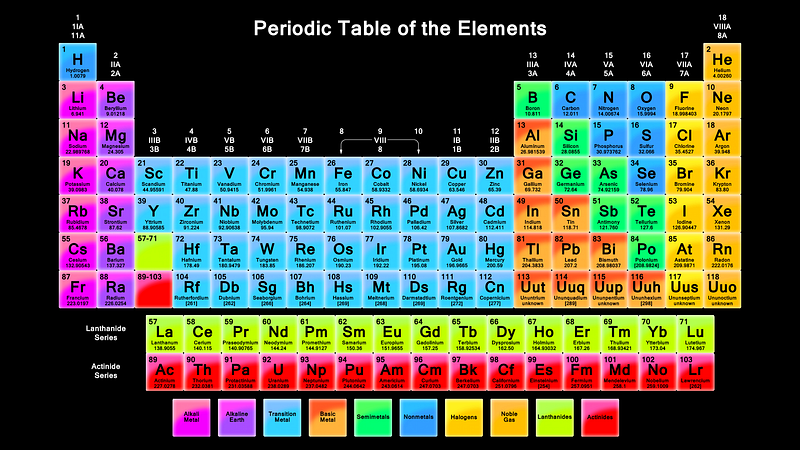
It’s these atoms that form the elements that all the material objects in the Universe we’re familiar with are made of. This includes everything from single atoms to simple molecules to complex macromolecules and molecular chains, all the way up to organelles, cells, specialized organs and entire functioning organisms.
Everything found on Earth is made out of this relatively small number of elements. As it turns out, elements one (hydrogen) through ninety-two (uranium) inclusive are found to occur naturally on our world, with two exceptions only: elements 43 (technetium) and 61 (promethium), which are radioactive in all forms on timescales far shorter than the Earth’s lifetime.

If we look into the depth of space, at interstellar gas clouds, at the surfaces of stars, and in the hearts of star-forming regions and supernova remnants, we can get a handle on just how common these elements are throughout our galaxy and the Universe. We find, perhaps unsurprisingly, that what we find on our planet’s crust is not a good representation of how abundant these various elements are, but what’s found in our Sun is mightily close. We can tell this by looking at the Sun’s absorption spectrum, and identify what elements (and in what ratio) are present.
In fact, if we graphed how abundant all the different elements are in our Solar System, you’d find what appears to be a nice pattern, with some ups-and-downs, but a general curve where the lightest elements are the most abundant, and the abundance of the heavier ones gradually decreases as we move farther and farther down the periodic table.
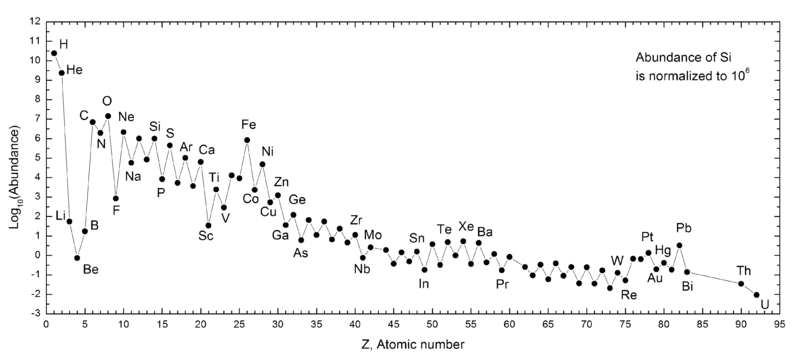
Or rather that general pattern seems to hold, if you forget about elements three, four and five in the periodic table: lithium, beryllium and boron! These three elements are virtually non-existent in the Sun (or any star), and look awfully peculiar when compared to all the elements around them.
On the other hand, it’s a good things they exist; lithium and boron may serve a biological purpose in humans, and boron is a necessity in the cell walls of all plants! These three elements are special in the Universe, and owe their origins to a different process than every other element in the periodic table.

In the very beginning, there were no elements; there was just a hot mix of quarks, gluons, electrons, neutrinos, radiation, unstable particles and antimatter. As the Universe expanded and cooled, though, the unstable particles decayed away, the antimatter annihilated with the matter (which there was just a little more of), and the quarks and gluons condensed into protons and neutrons. Initially, the Universe was too energetic for protons and neutrons to fuse together into heavier elements, as they’d immediately be blasted apart by the hot radiation.
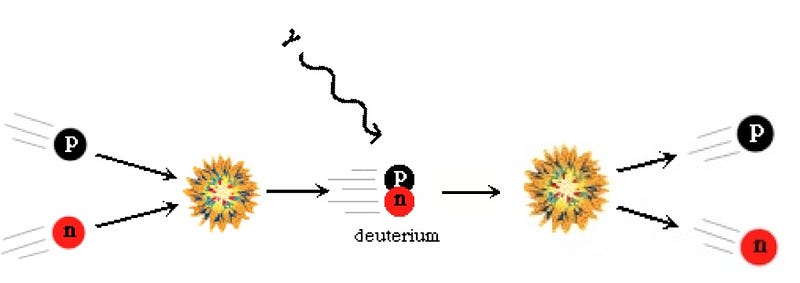
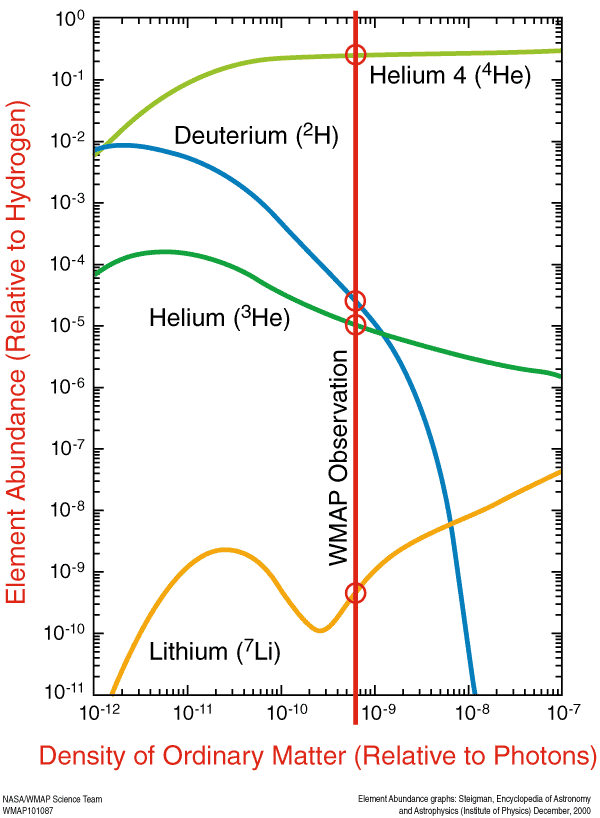
But as the Universe expanded and cooled, that radiation could no longer stop atomic nuclei from forming, and so the lightest elements in the Universe — hydrogen, helium, a couple of isotopes and a tiny bit of lithium — came into existence. Thanks to direct observations of these elements, a knowledge of the atomic-nuclei-to-photon ratio (from the microwave background), and a theoretical understanding of nucleosynthesis, we can see that our understanding matches up very well.
That takes care of the first two elements in the periodic table, but what about the rest? Well, we have stars! Undergoing nuclear fusion in their core, the Universe has had 13.8 billion years to create all the other elements. In the core of all main-sequence stars, hydrogen fuses into helium, and if your star is massive enough (and ours is), it will begin fusing helium into carbon, nitrogen and oxygen.

And in the most massive of stars, carbon can fuse into heavier elements, and then oxygen, and sulphur and silicon, and eventually you’re left with a core of iron, nickel and cobalt in a star that will go supernova in short order, creating all of the heavier elements in great abundance and spreading that material all over the Universe.

Over time, of course, the unstable elements will decay, and that’s why uranium is the heaviest naturally occurring element on Earth today. But what about that “gap” at the beginning? In the cores of stars, we went straight from helium to carbon, and just skipped over the three intermediate elements. In fact, if you put any lithium, beryllium or boron in a star, the high energies and temperatures of the star will destroy those elements, dissociating them into helium, hydrogen and possibly a few neutrons!
So where do these elements come from?
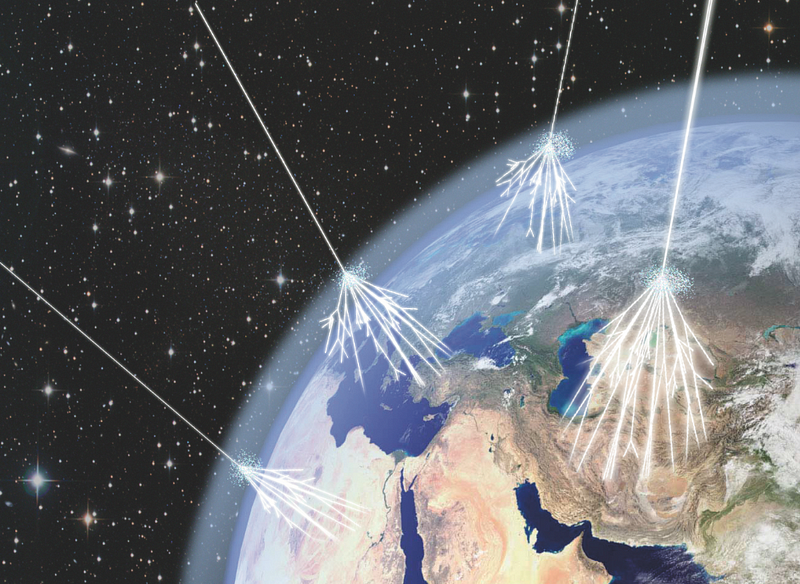
From the naturally accelerated particles flying through the Universe at nearly light speed: the cosmic rays! Produced by supernovae, active galaxies and probably neutron stars and black holes, these high energy protons and atomic nuclei (and the occasional electron) travel along in the Universe until some unlucky particle gets in the way, which it inevitably will.
And when that particle happens to be a carbon (or heavier) atom, look out!
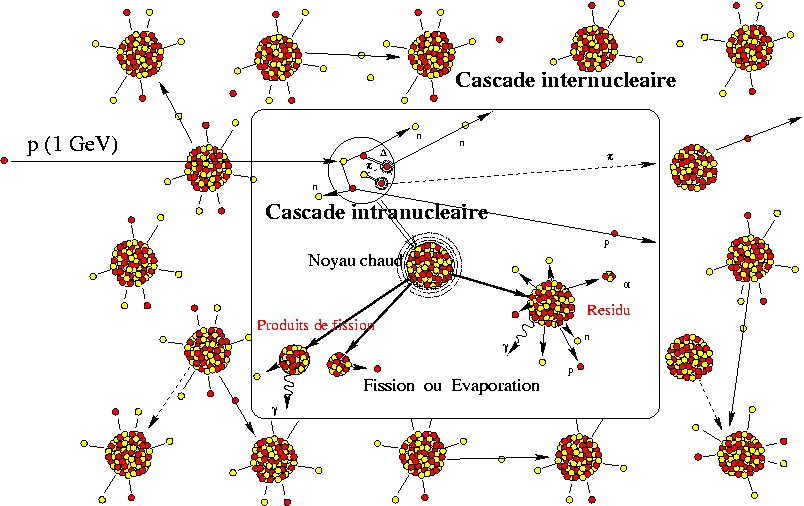
Because these cosmic rays can blast apart atomic nuclei into smaller constituents, via a process known as spallation.
While hydrogen (and a little bit of lithium) is produced in the Big Bang, carbon and heavier elements are produced in stars, and helium is produced in both, all the beryllium, boron, and most of the lithium found on Earth is produced by this process: of cosmic rays colliding with heavier, pre-existing atoms!
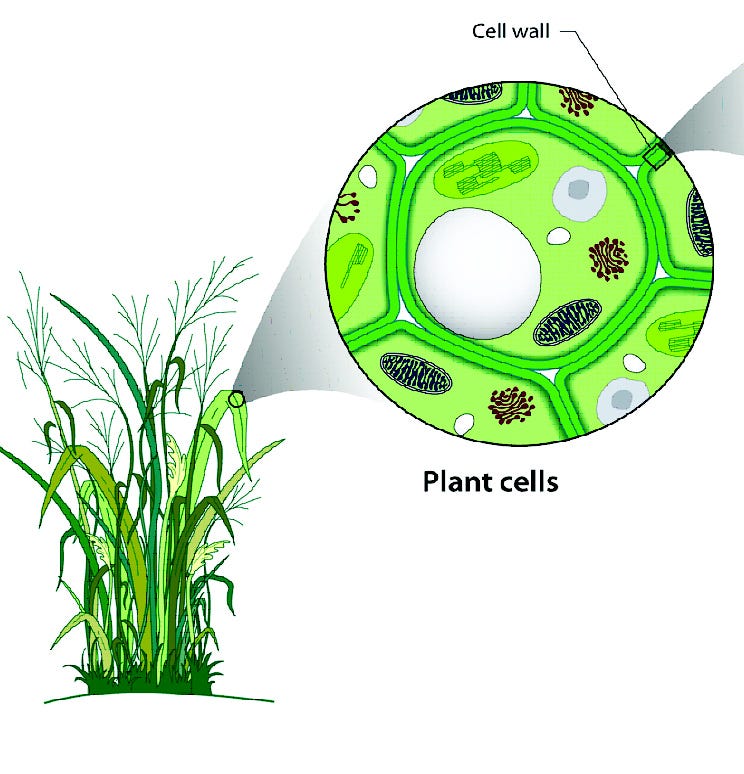
So the next time you look at a plant, and you examine the outer wall of its cells, think about the fact that the atoms that give these cells their unique properties — the boron atoms — needed a particle accelerated by a black hole, neutron star, supernova, or distant galaxy to collide with the heavy elements expelled from a prior generation of stars.

And then, it had to not find its way into another star before coming to us! And that’s the unique story of the three rarest light elements in the Universe: lithium, beryllium and boron.
Enjoyed this story? Weigh in at the Starts With A Bang forum on Scienceblogs!