This Is Why Mars Is Red And Dead While Earth Is Blue And Alive

The two planets most suited for habitability had very different fates. At last, scientists know why.
Imagine the early days of our Solar System, going back billions of years. The Sun was cooler and less luminous, but there were (at least) two planets — Earth and Mars — with liquid water covering large portions of their surfaces. Neither world was completely frozen over owing to the substantial presence of greenhouse gases, including carbon dioxide. Both may have even had primitive life forms in their young oceans, paving the way for a bright, biology-friendly future.
Over the past few billion years, both planets have undergone dramatic changes. Yet, for some reason, while Earth became oxygen-rich, remained temperate, and saw life explode on its surface, Mars simply died. Its oceans disappeared; it lost its atmosphere; and no life signs have yet been found there. There must be a reason why Mars died while Earth survived. It took decades, but science has finally figured it out.

One of the most spectacular features of Earth is the fact that the history of life on our world is written into the fossil record. Over hundreds of millions of years, sediments have been deposited both on land and in the oceans, with various organisms leaving their telltale imprints within them.
Of all the sedimentary rocks on Earth, about 10% of them are limestone, which are often composed of the remnants of marine organisms like coral, amoebas, algae, plankton, and mollusks. Limestone is primarily made of calcium carbonate, while some forms also have magnesium and silicon present.

The “carbonate” part, however, is universal to limestone on Earth, as well as other ocean-deposited minerals like the magnesium-rich dolomite. It’s the carbon dioxide in the atmosphere that leads to the formation of carbonate rocks, as
- the gaseous CO2 in the atmosphere gets soaked up by the ocean until there’s an equilibrium point reached,
- and then that oceanic carbon dioxide combines with minerals (such as calcium, magnesium, etc.) found in the water,
- either forming grains or chemical precipitates,
- that then get deposited on the ocean floor, leading to sedimentary rock formation.
There are both biological and geochemical origins for the limestone we find on Earth, making it one of the most abundant rocks on Earth’s surface. It’s generally thought that the vast majority of Earth’s early CO2atmosphere eventually wound up in our surface limestone.

There is an overwhelming amount of evidence that Mars had a watery past. Seasonal ices can be found not only at the poles, but in various basins and craters dotting the Martian surface. Features like dried-up riverbeds — often featuring oxbow bends like those found on Earth — stream throughout the landscape. Evidence of ancient flows leading into great oceanic basins, possibly even including tidal rhythmites, abounds all over the red planet.
These features may have been telltale signs of an ancient past where liquid water was abundant, but that’s no longer the case today. Instead, there’s so little atmosphere left on Mars that pure, uncontaminated liquid water is actually impossible at most locations on Mars. There’s simply insufficient pressure at the surface for liquid H2O to exist.

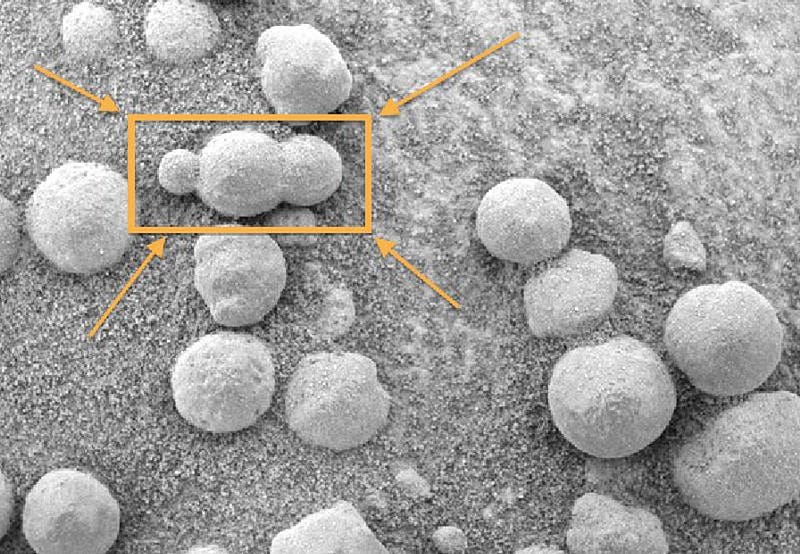
Even before we had rovers exploring the surface of Mars, the evidence of a watery past was very strong. Once we began exploring the surface in earnest, however, the evidence became too strong to ignore. The hematite spheres found by the Mars Opportunity rover all but sealed it. Particularly with the way some of the spheres were seen to be connected to one another, there was no reasonable possibility of forming them without liquid water.
Since Mars once had a similarly CO2-rich atmosphere to early Earth, it was assumed that limestone and other carbonate rocks would be found on its surface. But there was none found by the Viking landers, nor by Soujourner, Spirit, or Opportunity.
It wasn’t until the Mars Phoenix lander arrived that any calcium carbonate was found at all, and even that was a small amount: likely produced by an evaporating body of water in its final stages. Compared to the hundreds of meters (or even in excess of a kilometer in places) of carbonate rocks on Earth, there was nothing like it on Mars.
This was extraordinarily puzzling to Martian scientists. Perhaps 20 years ago, the overwhelming expectation was that Mars would have lost its carbon dioxide the same way Earth did: to its oceans and then to deposition in carbonate rocks. But that’s not what the rovers found. In fact, in place of carbonates, they found something else that was perhaps equally surprising: sulfur-rich minerals. In particular, it was Opportunity’s discovery of the mineral jarosite that completely changed the story.

This allowed scientists to paint an entirely different picture of Mars from Earth. On Earth, our oceans are approximately pH-neutral, which is extremely conducive to carbonate rocks precipitating out. Even in a CO2-rich environment, the carbonic acid still leads to a pH that’s high enough that carbonates will precipitate out, leading to the limestones and dolomites found all over Earth’s surface.
But sulfur changes the story dramatically. If early Mars had an atmosphere rich in not just carbon dioxide but also sulfur dioxide, its surface water could have been affected not by carbonic acid, but by sulfuric acid: one of the strongest acids in all of chemistry. If the oceans were acidic enough, it could have engineered the reverse reaction to what happened on Earth: sucking carbonates out of the land and into the oceans, leaving sulfur-rich deposits in their place.

This would explain the ocean and surface chemistry of Mars, but would mean we needed an entirely different mechanism to explain where the Martian atmosphere went. Whereas a large portion of Earth’s atmosphere ended up in the Earth itself, that explanation simply wouldn’t fly for Mars.
Instead of “down,” perhaps the atmosphere went “up” and into the depths of space.
Perhaps Mars, much like Earth, once had a magnetic field to protect it from the solar wind. But at just half the diameter of Earth and with a lower-density, smaller core, perhaps Mars cooled enough so that its active magnetic dynamo went quiet. And perhaps this was a turning point: without its protective magnetic shield, there was nothing to protect that atmosphere from the onslaught of particles from the Sun.
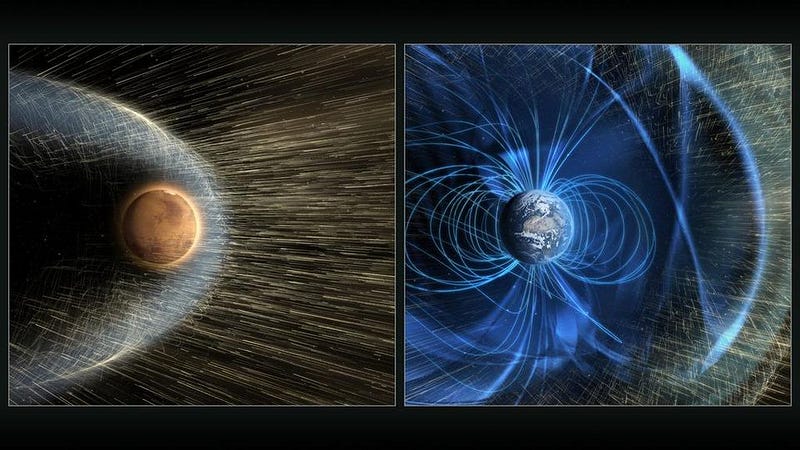
Was this correct? Is this really how Mars lost its atmosphere, stripping the planet of its ability to have liquid water at the surface and rendering it cold, sparse and barren?
That was the whole purpose behind NASA’s MAVEN mission. The goal of MAVEN was to measure the rate at which the atmosphere was being stripped by the solar wind from Mars today, and to infer the rate throughout the red planet’s history. The solar wind is powerful, but molecules like carbon dioxide have a high molecular weight, meaning it’s difficult to get them up to escape velocity. Could the loss of a magnetic field coupled with the solar wind provide a viable mechanism to transform Mars from an atmosphere-rich world with liquid water at its surface to the Mars we know today?
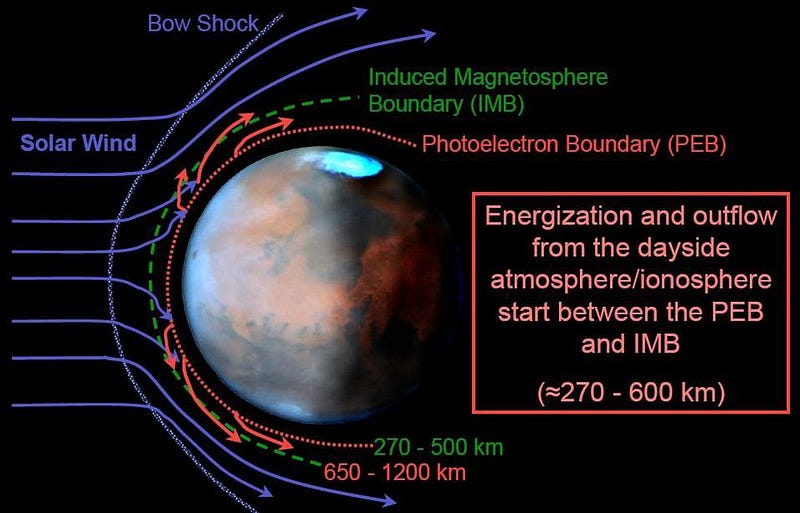
What MAVEN saw was that Mars loses, on average, about 100 grams (¼ pound) of atmosphere to space every second. During flaring events, where the solar wind becomes much stronger than normal, that increases to about twenty times the typical value. When the atmosphere was much denser, though, the same level of solar wind would strip it away much more quickly.
Timescales of merely ~100 million years would be sufficient to transform a Mars-sized world, without any protection from the solar wind, from having an Earth-like atmosphere to one akin to what we find on present-day Mars. After perhaps a billion years with liquid water precipitating and flowing freely on the Martian surface, a tiny slice of cosmic history was enough to blow the habitable prospects of Mars completely away.
Both Mars and Earth had early atmospheres that were heavy, massive, and extraordinarily rich in CO2. While Earth’s carbon dioxide got absorbed into the oceans and locked up into carbonate rocks, Mars was unable to do the same, as its oceans were too acidified. The presence of sulfur dioxide led to Martian oceans that were rich in sulfuric acid. This led to geology of Mars we’ve discovered with rovers and landers, and pointed to a different cause — the solar wind — as the culprit in the mystery of the missing Martian atmosphere.
Thanks to NASA’s MAVEN mission, we’ve confirmed that this story is, in fact, the way it happened. Some four billion years ago, the core of Mars became inactive, its magnetic field disappeared, and the solar wind stripped the atmosphere away. With our magnetic field intact, our planet will remain blue and alive for the foreseeable future. But for a smaller world like Mars, its time ran out long ago. At last, we finally know why.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.