Is the Universe fundamentally unstable?

- There is no question more important to the long-term fate of our Universe, especially given the presence of dark energy, than the stability of the quantum vacuum.
- If it's inherently stable, then dark energy can maintain its current value and the laws of physics can remain the same arbitrarily far into the future; our fate will be an eventual heat death.
- But if it's unstable, then the quantum vacuum may decay to a more stable. lower-energy state. If this occurs, our Universe will fundamentally change, and our end will be swift, brutal, and terrifying.
There are certain properties about the Universe that for better or worse we take for granted. The laws of physics, we presume, are the same at other locations in space and other moments in time as they are in the here-and-now. The fundamental constants that relate various physical properties of our Universe are assumed to truly possess the same, constant value at every time and place. The fact that the Universe appears to be consistent with these presumptions — at least, to the limits of our observations — seems to support this view, placing great constraints on how much it’s possible these various aspects of reality have evolved.
Wherever and whenever we can measure or infer the fundamental physical properties of the Universe, it appears that they do not change over time or space: they are the same for everybody. But earlier on, the Universe underwent transitions: from higher-energy states to lower-energy ones. Some of the conditions that arose spontaneously under those high-energy conditions could no longer persist at lower energies, rendering them unstable. Unstable states all have one thing in common: they decay. And in one of the most terrifying realizations of all, we’ve learned that the fabric of our Universe itself may inherently be one of those unstable things as well. Here’s what we know, today, about how precarious our continued existence is.

In any physical system — that is, a system made up of particles that interact via one or more forces — there’s at least one way to configure them that is more stable than any other way to do it. This is what we call the lowest-energy state, or the ground-state, of a system.
- Planets organize themselves into a spheroidal shape that represents hydrostatic equilibrium, with denser elements toward the center and less dense elements toward the outskirts. They tend toward more-stable states over time, as well, as each large earthquake changes the distribution of the Earth’s mass, causing its rotation to speed up as a side-effect.
- Planets within stellar systems typically organize themselves into resonant, nearly-circular orbits, as their mutual gravitational influences “iron out” imperfections over time, sometimes at the cost of gravitationally ejecting one or more members.
- And balls placed on a hilly surface will tend to roll down into the valley below, coming to rest at the bottom: at the lowest possible elevation that their initial conditions enabled them to reach.
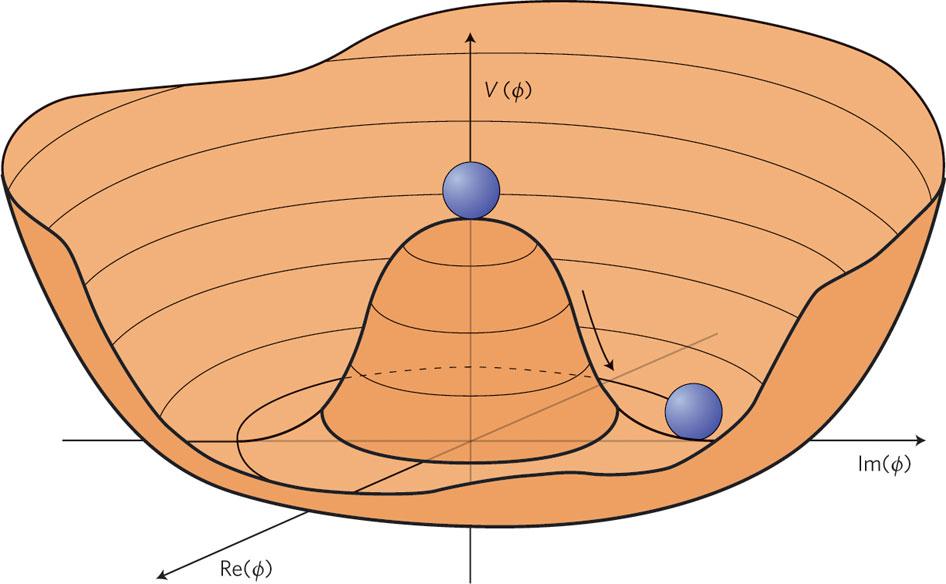
Only, that last example has a catch to it: sometimes, if your conditions aren’t precisely right, your ball won’t end up in the lowest-energy state possible. Rather, it can roll into a valley that’s still lower than where it started, but that doesn’t represent the true ground state of the system. This state can happen naturally for a great variety of physical systems, and we generally think about it as though the system is “hung up” in some sort of false minimum. Even though it would be more energetically stable in the ground state, or in its true minimum, it can’t necessarily get there on its own.
What can you do when you’re stuck in a false minimum?
If you’re a classical system, the only solution is Sisyphean: you have to input enough energy into your system — irrespective of whether that’s kinetic energy, chemical energy, electrical energy, etc. — to “kick” that system out of the false minimum. If you can overcome the next energy barrier, you have the opportunity to wind up in an even more stable state: a state that takes you down closer to, and possible even all the way to, the ground state. Only in the true ground state is it impossible to transition down to an even lower-energy state.
That’s what’s true for a classical system. But the Universe isn’t purely classical in nature; rather, we live in a quantum Universe. Inherently quantum systems not only undergo these same types of reorganizations as classical systems — where inputting energy can kick them out of unstable equilibrium states — but they have another effect that they’re subject to: quantum tunneling.
Quantum tunneling is a probabilistic venture, but one that doesn’t require what you might think of as “activation energy” to get over that hump keeping you in that unstable equilibrium state. Instead, dependent on specifics like how far your field is from the true equilibrium state and how high the barrier is preventing you from leaving the false minimum that you’re stuck in, there’s a certain probability that you can spontaneously leave your unstable equilibrium state and find yourself, all of a sudden, in a more stable (or even the true) minimum of your quantum system.
Unlike in the purely classical case, this can happen spontaneously, with no outside, energetic influence or impetus required.

Some common examples of quantum systems that exhibit tunneling involve atoms and their constituent particles.
- Electrons within atoms, for example, often find themselves in an excited state: where they’re in a higher energy level other than the ground state. Often, that’s because other electrons are in those lower-energy states; if they’re all occupied, then that electron is in its lowest-energy configuration. Sometimes, there are “openings” in those lower-energy states, and those higher-energy electrons will spontaneously cascade downward, emitting energy in the process. But other times — due to subtle effects like spin-orbit interactions or hyperfine splitting — there is a more stable state, but the spontaneous pathway is forbidden by the rules of quantum mechanics. Nevertheless, you can still leave the unstable equilibrium state and arrive at the ground state via quantum tunneling: the source of the famed 21-cm hydrogen line.
- Atomic nuclei, composed of protons and neutrons, always have a most-stable configuration for any unique number of protons and neutrons composing that nucleus. For very heavy nuclei, however, sometimes that nucleus would be more stable if one of its neutrons radioactively decayed, or if it emitted a helium-4 nucleus (with 2 protons and 2 neutrons), and then reconfigured itself into a new arrangement. These inherently probabilistic quantum decays also spontaneously tunnel from a less stable to a more stable state.

Well, you know what the ultimate quantum system is?
Empty space itself. Empty space — even without any particles, quanta, or external fields present — still appears to have a non-zero amount of energy inherent to it. This evidences itself through the observed effects of dark energy, and even though it corresponds to a very small energy density of barely more than a proton’s worth of energy per cubic meter of space, that’s still a positive, finite, non-zero value.
We also know that regardless of how much you remove from any particular region of space, you cannot get rid of the fundamental quantum fields that describe the interactions and forces inherent to the Universe. Just as you cannot have “space” without the laws of physics, you cannot have a region without the presence of quantum fields owing to (at least) the forces of the Standard Model.
It had long been assumed, although it was untested, that because we do not know how to calculate the energy inherent to empty space — what quantum field theorists call the vacuum expectation value — in any way that doesn’t yield complete nonsense, it probably all just cancels out. But the measurement of dark energy, and that it affects the expansion of the Universe and must have a positive, non-zero value, tells us that it cannot all cancel out. The quantum fields permeating all of space give a positive, non-zero value to the quantum vacuum.

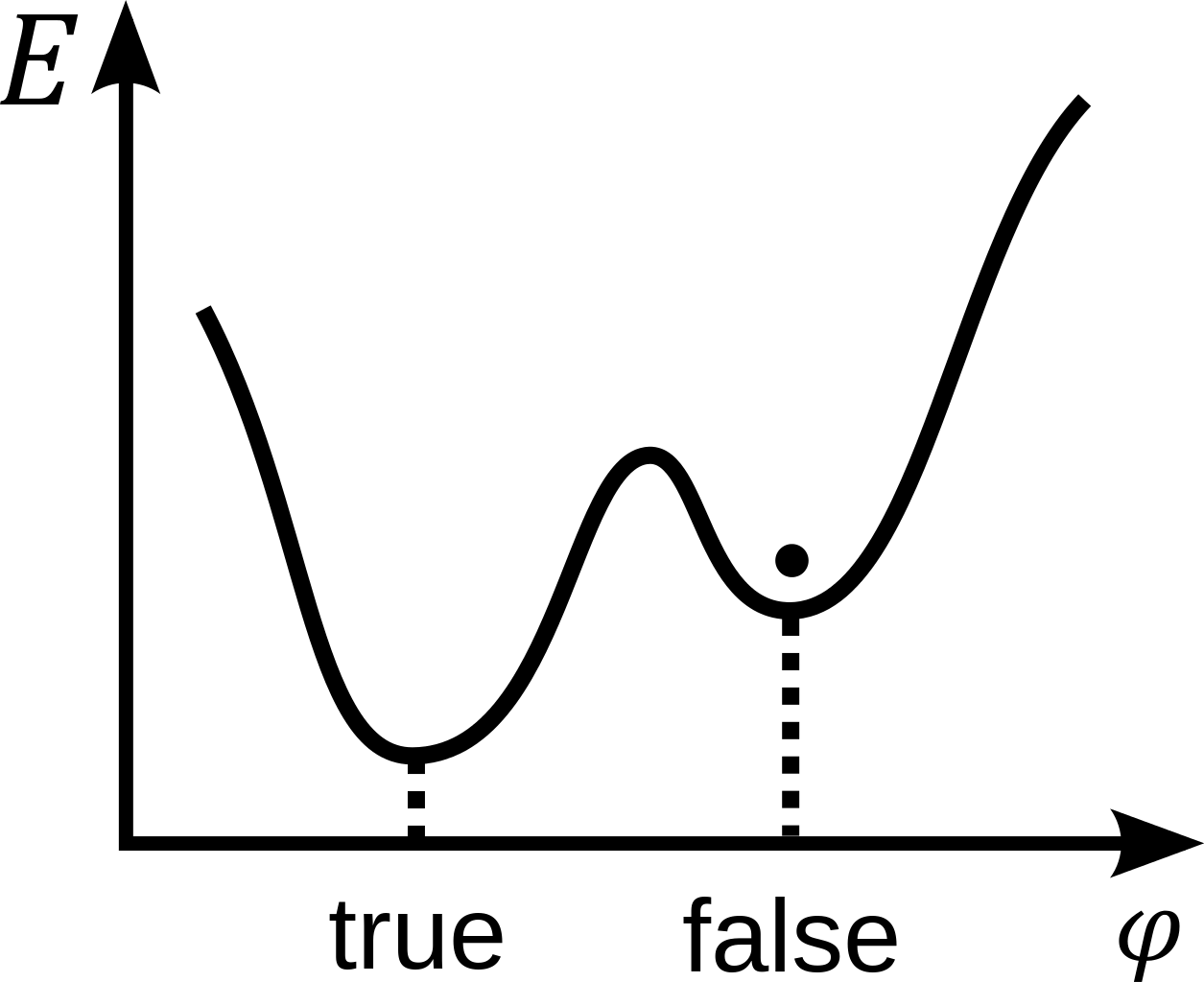
Now, here’s the big question: is the value that we’re measuring for dark energy, today, the same value that the Universe recognizes as its “true minimum” for the contributions of the quantum vacuum to the energy density of space?
If it is, then great: the Universe will be stable forever and ever, as there’s no lower-energy state for it to ever quantum tunnel into.
But if we’re not in a true minimum, and there is a true minimum out there that actually represents a more stable, lower-energy configuration than the one we currently find ourselves (and the entire Universe) in, then there’s always a probability that we’ll eventually quantum tunnel into that true vacuum state.
This latter option, unfortunately, is not so great. The vacuum state of the Universe, remember, depends on the fundamental laws, quanta, and constants that underlie our Universe. If we spontaneously transitioned from our current vacuum state to a different, lower-energy one, it isn’t just that space would now take on a different configuration. In fact, by necessity, we’d have at least one of:
- a different set of physical laws,
- a different set of quantum interactions that could occur,
- and/or a different set of fundamental constants.
If this change were to spontaneously occur, what happened next would be a Universe-ending catastrophe.

Wherever the quantum vacuum transitioned from this false vacuum state into the true vacuum state, everything that we recognize as a bound state of quanta — things like protons-and-neutrons, atomic nuclei, atoms, and everything that they make up, for example — would immediately be destroyed. As the fundamental particles that compose reality rearrange themselves according to these new rules, everything from molecules to planets to stars to galaxies would come undone, including human beings and any living organisms.
Without knowing what the true vacuum state is and what these new sets of laws, interactions, and constants our current ones would be replaced with, we have no way of predicting what sorts of new structures would emerge. But we can know that not only would the ones we see today cease to exist, but that wherever this transition occurred, it would propagate outward at the speed of light, “infecting” space as it expanded with a great bubble of destruction. Even with the Universe expanding, and even with that expansion accelerating due to dark energy, if a vacuum decay event such as the one envisioned here occurred anywhere within 18 billion light-years of us, at present, it would eventually reach us, destroying every atom at the speed of light in a Ghostbusters-level event when it did.
Is this something we actually have to worry about?
Maybe. There are consistency conditions that must be obeyed by the laws of physics, and there are parameters that we need to measure in order to find out whether we live in a:
- stable Universe, whose quantum vacuum will never decay,
- an unstable Universe, whose quantum vacuum should decay immediately,
- or a meta-stable Universe, where we’re in precisely one of these “false minima” that could someday decay to the true minimum.
In the context of quantum field theory, this means that if we take the properties of the Standard Model, including the particle content of the Universe, the interactions that exist between particles, and the relationships that govern the overarching rules, then we can measure the parameters of the particles within it (such as the rest masses of the particles), and determine what type of Universe we live in.
Right now, the two most important parameters in performing such a calculation are the mass of the top quark and the Higgs boson. The best value we have for the top mass is 171.77±0.38 GeV, and the best value we have for the Higgs mass is 125.38±0.14 GeV. This appears extremely close to the metastable/stable border, where the blue dot and the three blue circles below represent 1-sigma, 2-sigma, and 3-sigma departures from the mean value.
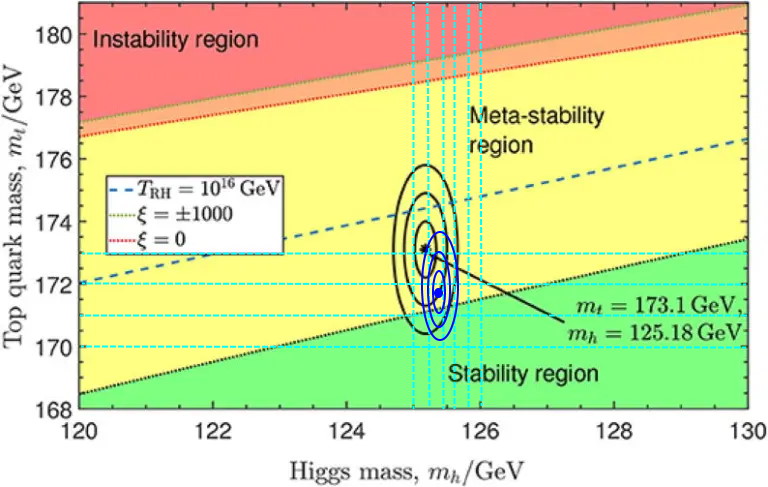
Does this mean the Universe is really in a metastable state, and the quantum vacuum may actually someday decay where we are, ending the Universe in a catastrophic fashion that’s very different from the slow, gradual heat death we’d otherwise expect?
That depends. It depends on which side of that curve we’re on, and that depends on whether we’ve correctly identified all of the underlying laws of physics and the contributors to the quantum vacuum, whether we’ve done our calculations correctly assuming we’ve written down the underlying equations properly, and whether our measurements for the masses of the constituent particles of the Universe are accurate and precise. If we want to know for certain, we know at least this much: we need a better determination of these measurable parameters, and that means creating more top quarks and Higgs bosons, measured to at least the best precision we can currently muster.
The Universe may fundamentally be unstable, but if it is, we’ll never see this bubble of destruction caused by vacuum decay coming our way. No information-carrying signal can travel faster-than-light, and that means that if the vacuum does decay, our first warning of its arrival will coincide with our instantaneous demise. Nevertheless, if our Universe truly is fundamentally unstable, I’d want to know. Would you?