Why can hot water freeze faster than cold?
It’s known as the Mpemba effect, and it’s been observed as far back as Aristotle. But hot water really can sometimes freeze faster than cold, and the science of why is only beginning to be fully understood.
“I have lived in a flurry of images, but I will go out in a freeze frame.” -Anthony Quinn
You’ve all seen the footage and heard of the trick by now: throw a pot of boiling water in the air and watch it turn into snow. (Assuming you don’t burn yourself instead.) The physics behind this weird behavior is incredibly interesting and related to what happens to water in space, but there’s an even more bizarre and counterintuitive phenomena out there, as my old college friend Ricardo asks:
In some circumstances, warmer water can freeze faster than colder water. Why is this?
This is known as the Mpemba effect, and believe it or not, it’s real.

The effect is named after a Tanzanian schoolchild, Erasto Mpemba, who noticed while making ice cream with his classmates that warm milk froze faster than cold milk. Although this type of effect has been observed many times historically, on the surface it hardly makes any sense. Let’s think about why.
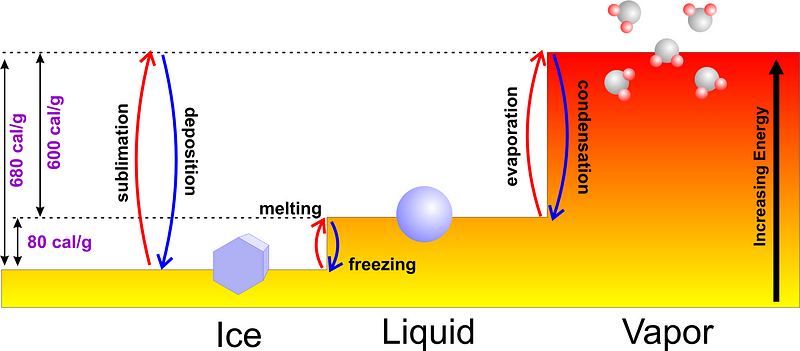
Normally, if you start with liquid water, you can either add energy to it, heating it to its boiling point of 100 °C (212 °F) and continuing to add energy as it goes through its phase change into water vapor, or you can remove energy from it, cooling down to its freezing point of 0 °C (32 °F) and continuing to remove heat as you turn it into ice. It would only make sense that if you began with colder water, it would freeze faster, since it would take less time to reach the freezing point in the first place!
In fact, most experiments that you can do will show exactly this: the colder water freezes first.
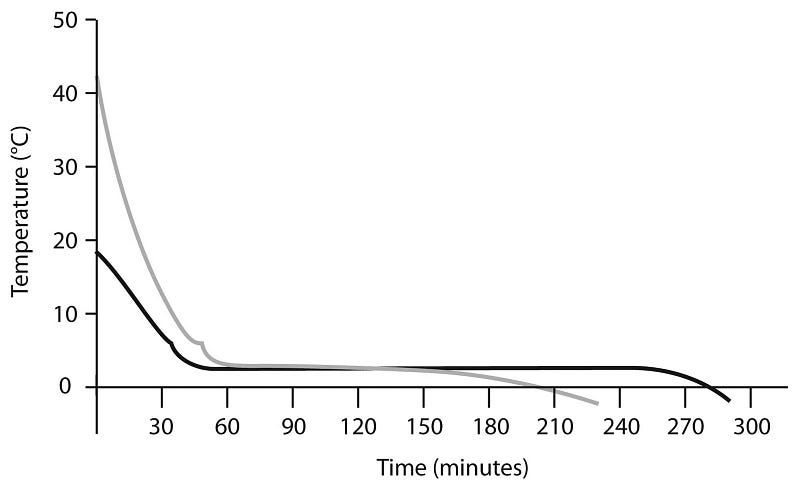
Even a “failed” experiment like this, however, offers a clue to how hot water might freeze faster than cold: notice how much faster the hot water cools down than the cold! Again, this is very intuitive, since if you put a 10 °C pot of water and a 90 °C pot of water in -10 °C surroundings, the one with the 100 °C temperature difference is going to lose heat a lot more quickly than the one with just a 20 °C difference.
But there’s a little more to the story than that, and it has everything to do with the unique properties of water.
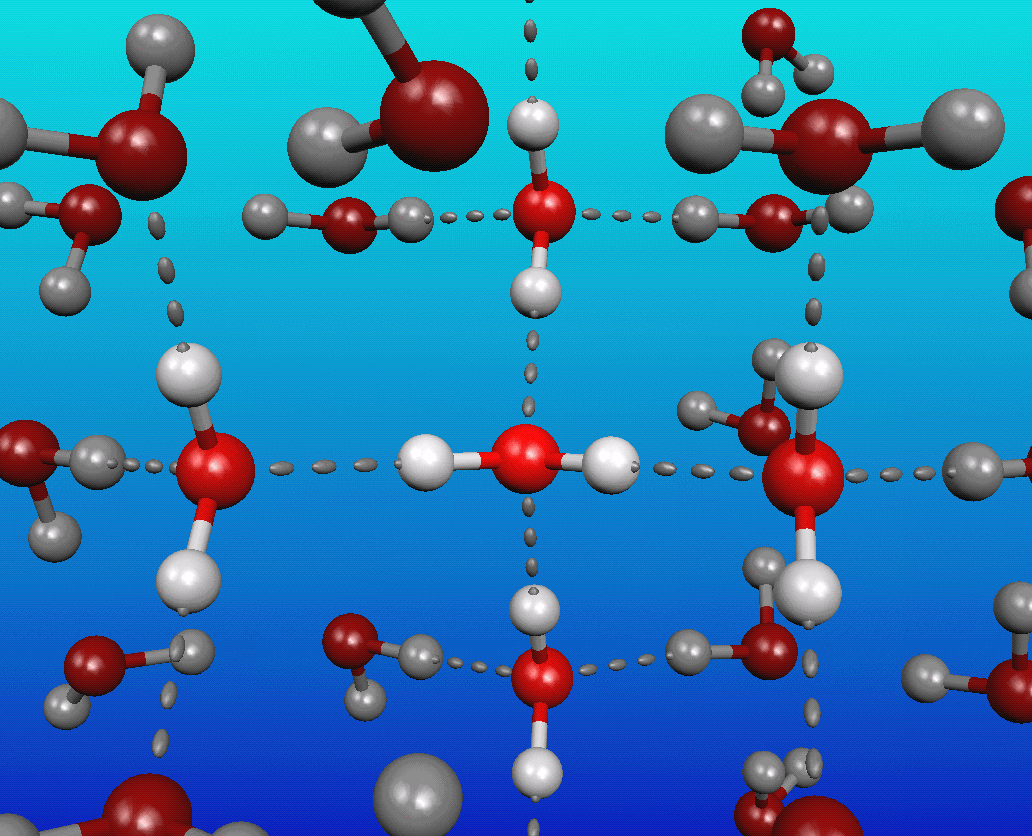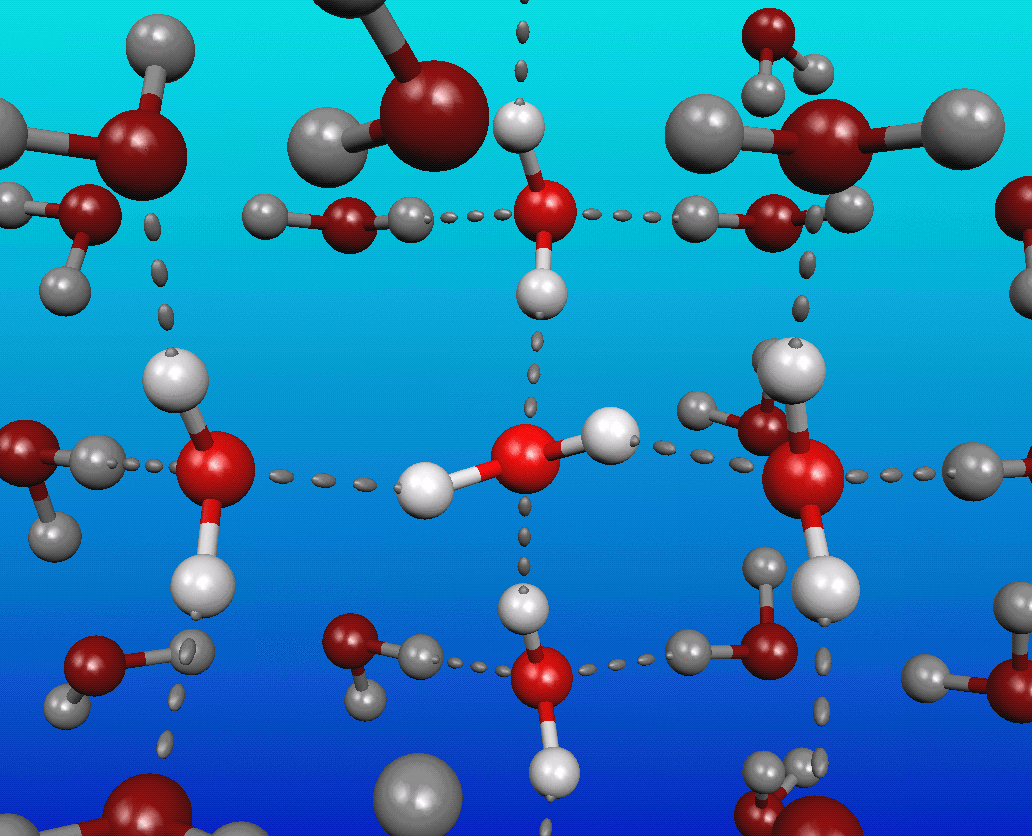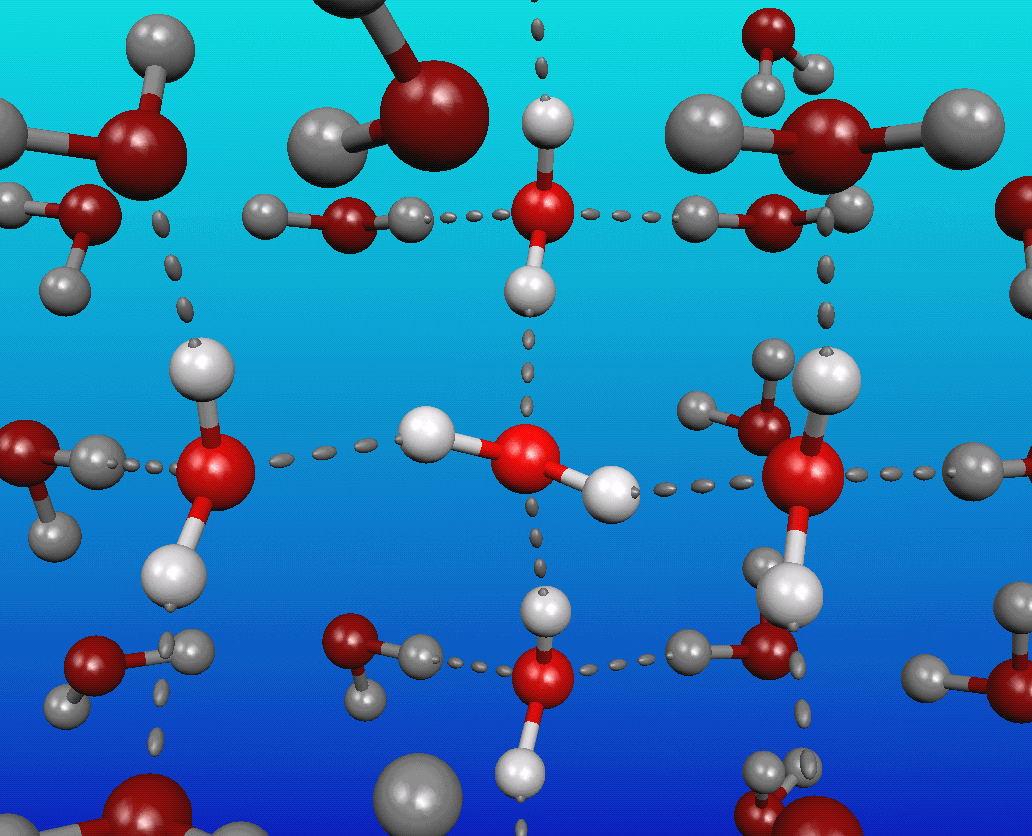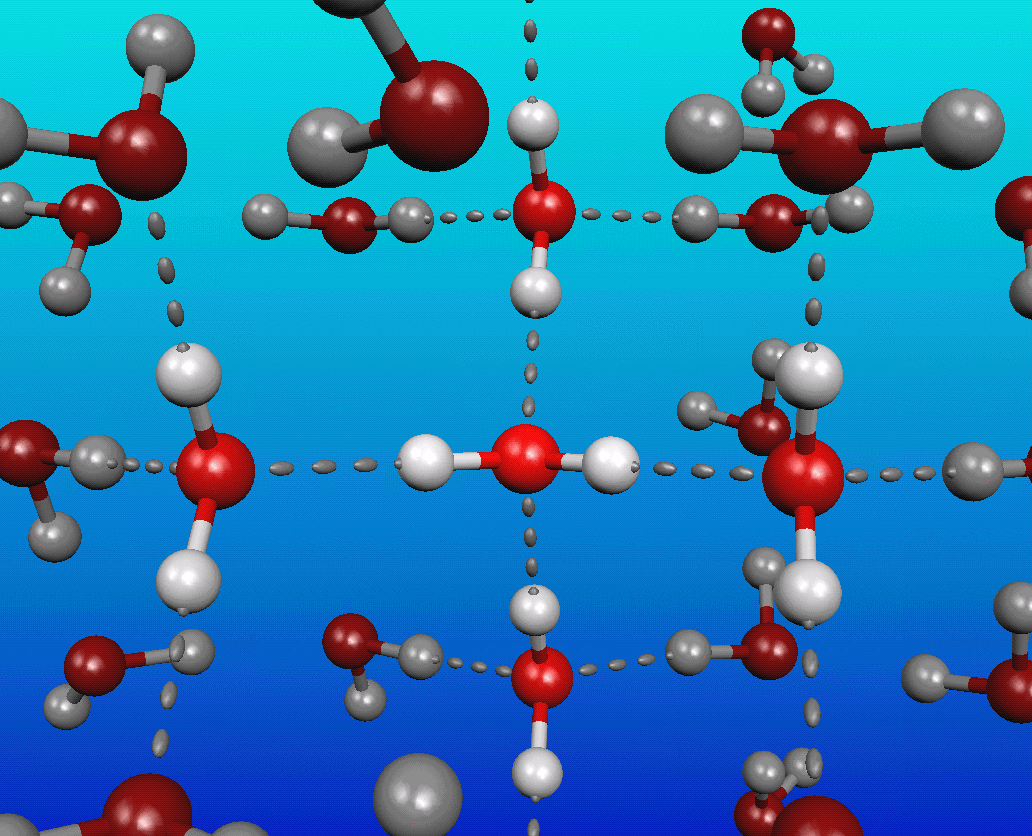
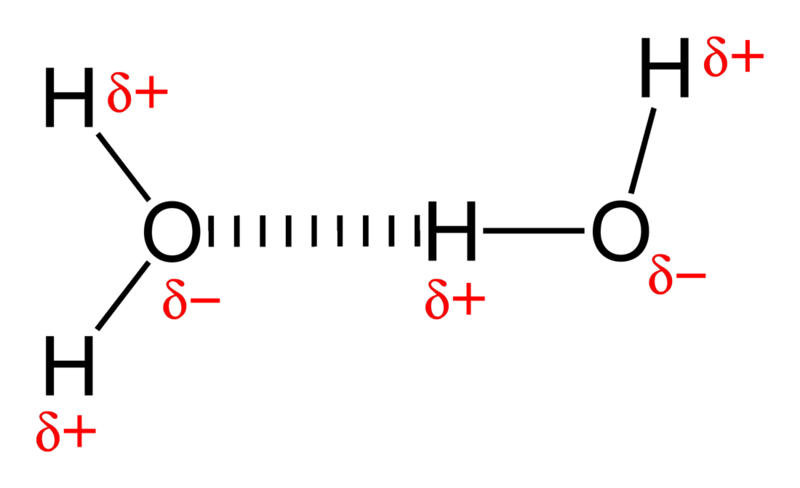
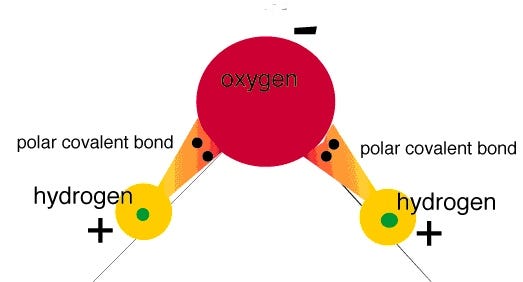
You see, water is a very polar molecule, with an extremely electronegative oxygen atom attached to two hydrogens. In chemistry-speak, each oxygen is an electron hog, meaning that portion of the molecule is generally negatively charged, while the hydrogen side is electron-deficient, leaving it positively charged.
And if you take molecules that have negative-and-positive ends and put a whole bunch of them together, they’re going to form loose bonds with one another; this is known as hydrogen bonding.
Now in the liquid phase, water molecules are free to rotate and move around some, much moreso than in the solid phase but not nearly as much as in the gaseous phase. But what do you think happens when you have hot water versus cold water?
You’re probably familiar with this childhood experiment: dropping food coloring into both hot water and cold water.
The hotter the water is, the faster the individual molecules can race around and disperse. What’s going on at a molecular level is that the hotter any substance is:
- the faster your molecules move around,
- the easier it is to spontaneously break those tenuous, intermolecular hydrogen bonds, and
- the more the covalent bonds in each molecule itself lengthen.
At least, those three things are what typically happen for a liquid substance. But water is just a little bit unusual.
The funny thing about it is that at cold (low) temperatures, each molecule of water typically has at least four neighboring water molecules, each tugging on this highly polar molecule. These neighboring molecules — even with their weak hydrogen bonds — effectively stretch the covalent bonds between the hydrogen and oxygen atoms.

This roughly tetrahedral structure around each water molecule is highly disrupted in hotter water, meaning that there’s no longer this inter-molecular stretching going on. So while the water molecules are moving around faster, and it is easier to break these tenuous hydrogen bonds, the covalent bonds inside each water molecule actually shrink as they increase in temperature!
So of the three standard things that typically happen for liquids, two happen for water, but the opposite happens for the third! So for hot water, these covalent bonds are shorter and stiffer, and when you cool it, contracting the hydrogen bonds, that forces the covalent bonds to lengthen, which means a faster relaxation time and — under the right conditions — a faster arrival at the freezing point for initially hotter water!
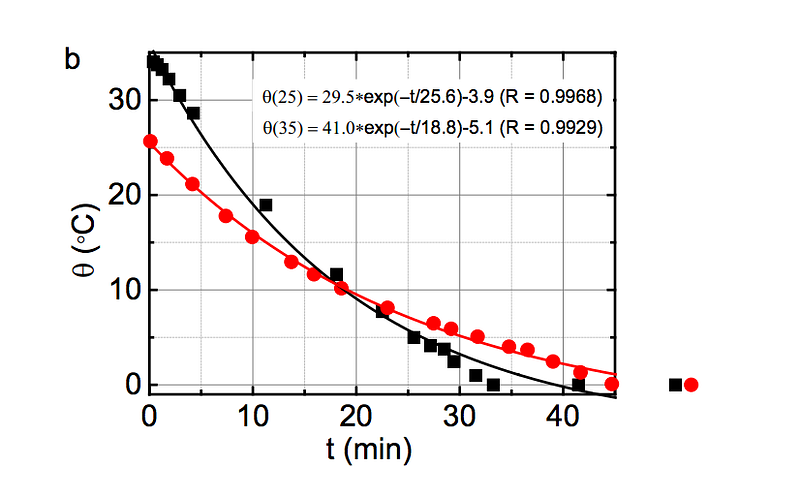
The higher temperature the water starts off at, the more energy is stored in those shorter, stiffer covalent bonds, and then placing that water in a very cold environment causes energy to be released at a rate that is exponentially dependent on the initial bond energy!
(Below to the left, you can see how τ, the energy release timescale, is much shorter at higher initial temperatures, and at right, you can see how the energy of the covalent bond is greater at higher initial temperatures.)
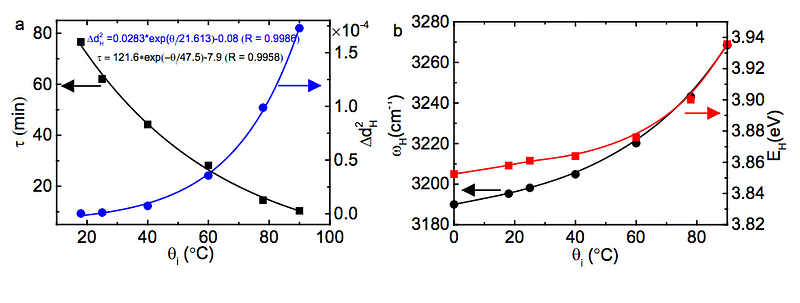
Experimentally, the best way to reproduce this result is to have relatively small amounts of cool water and water that’s nearly boiling as your initial subjects, and a “cold environment” that isn’t too much colder than freezing, but that’s large enough to be unaffected by the heat of the liquid water.
And that is our current understanding of why the Mpemba effect happens, or why initially hotter water can freeze faster than cold!





