Why the World Will Run Out of Helium
How the second most common element in the Universe is being lost from Earth, most of it for good.
“I have this one little saying, when things get too heavy just call me helium, the lightest known gas to man.” –Jimi Hendrix
Hendrix, as I told you once before, was almost right. We know of helium, conventionally, as the lighter-than-air gas that we fill balloons, blimps and zeppelins with in order to quickly and easily “defy gravity” here on Earth.

At least, defying gravity is what it appears to do. But what’s really going on is that helium is simply a very low-density gas. Our atmosphere, a mix of mostly Nitrogen (N2) and Oxygen (O2) gases, has an average molecular weight of 29. With a molecular weight of just four, Helium is bested only by pure Hydrogen (H2) gas (with a molecular weight of two) in the low-density department.
And just as a stone sinks in the ocean or olive oil floats atop a glass of water, the principle of buoyancy ensures that the least dense materials will eventually — once the effects of mixing are negligible (which they are, for atmospheric gases, over long enough times) — find themselves layered atop the progressively more dense materials.

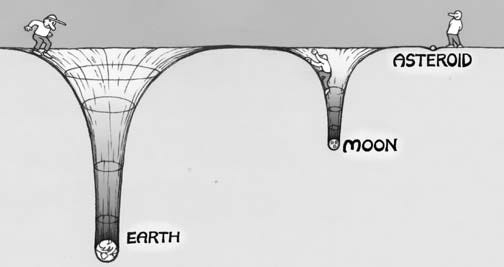
This is true for solids (such as in the interior of the Earth, where the core is denser than the mantle, which is denser than the crust, which is denser than the oceans and so on), liquids (as shown above), and gases as well. For a gas giant like Jupiter, because it’s so massive (many hundreds of times the mass of Earth), its gravitational well is tremendous, and even the lightest of its atmospheric gases stay bound to the planet itself. On the other extreme end, the Moon and asteroids are far too low in mass to hold on to any of their atmospheric gases, and even the heaviest gas will be expelled from the planet thanks to the intense radiation coming from our Sun.
The Earth’s atmosphere is today mostly composed of Nitrogen (78%), Oxygen (21%), with trace amounts of other gases. However, if we could go back to when the Solar System was first forming, we’d discover that every planet would have had an atmosphere loaded up with the two most common elements in the Universe: Hydrogen and Helium.
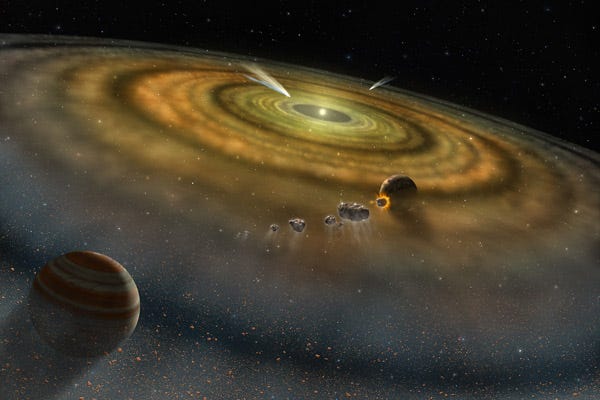
The vast majority of the Hydrogen and Helium would have been confined to the Earth’s atmosphere, and quickly would have risen to the uppermost layers. Other atoms — Carbon, Nitrogen, and Oxygen — would have bonded with Hydrogen (which is reactive) to produce methane, ammonia and water/water vapor, and not with Helium (which is inert). While the vast majority of the pure hydrogen gas and the helium would have been kicked off the planet relatively quickly by the Sun, the remaining gases would have stuck around for much longer, eventually leaving behind only the heaviest components of our atmosphere.
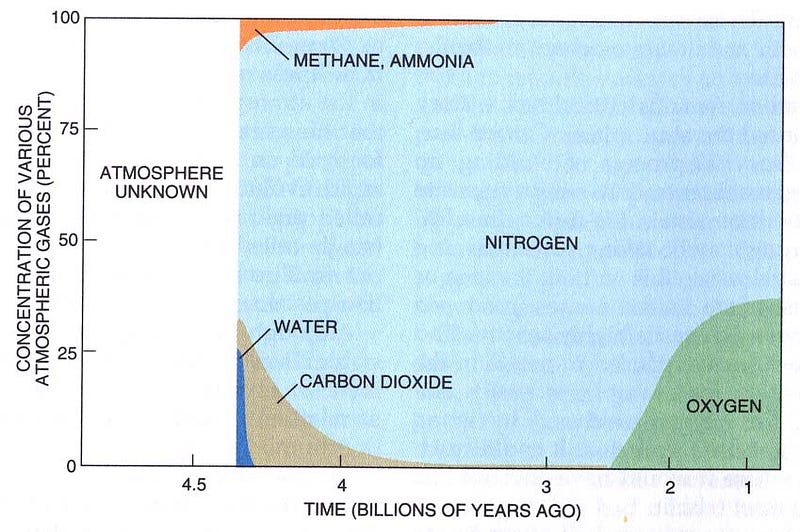
Now, it’s no problem to “make” hydrogen, we’ve got oceans full of material that’s 2/3 hydrogen. But what about Helium?
From the birth of the Solar System, there’s practically no Helium left on Earth. (To be more precise, Helium composes 0.00052% of the Earth’s atmosphere by volume.) Because of how large Earth’s atmosphere is, we could always try and mine that Helium from the atmosphere, but that process is tremendously difficult and expensive. But there is plenty of Helium here on Earth, and it has nothing to do with Helium in our atmosphere or, for that matter, anyplace else in the rest of the Universe.

These are some radioactive samples of Uranium and Thorium, two of the rarest elements found in nature. The most common isotopes — Uranium-238 and Thorium-232 — are radioactive, and will undergo a decay chain over timescales of billions of years.
Billions of years. In other words, a timescale comparable to the age of the Earth. Elements with much shorter lifetimes have all decayed away; that’s why Uranium (or maybe Plutonium) is the heaviest naturally occurring element here on Earth; while many others were certainly around immediately following the supernova explosion that triggered the formation of our Solar System, everything with shorter lifetimes is completely gone by now. But when Uranium-238 and Thorium-232 decay, they do so in a very particular and important fashion.
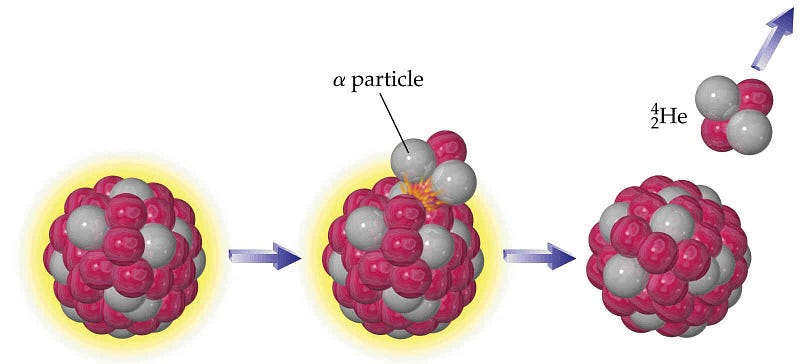
They undergo a process known as alpha decay, where heavy elements emit an alpha particle, eventually leading to the creation of a more stable nucleus. But the “alpha particle” — with two protons and two neutrons — is actually the nucleus of a Helium atom! It will very quickly (in a matter of microseconds) pick up a couple of electrons and become a neutral helium atom, and since Helium atoms don’t react with anything, all they’ll do is attempt to rise up to the top of the atmosphere.
Which they can only do if they can find the atmosphere! Most of the Uranium and Thorium in the world exists deep underground, so wherever there’s a Uranium or Thorium deposit in the Earth’s crust that’s been there for a long time (like, hundreds of millions of years), you’re bound to find a large deposit of Helium gas, too. Luckily (perhaps) for Earth, the largest one in the world sits squarely underground near the middle of the United States.
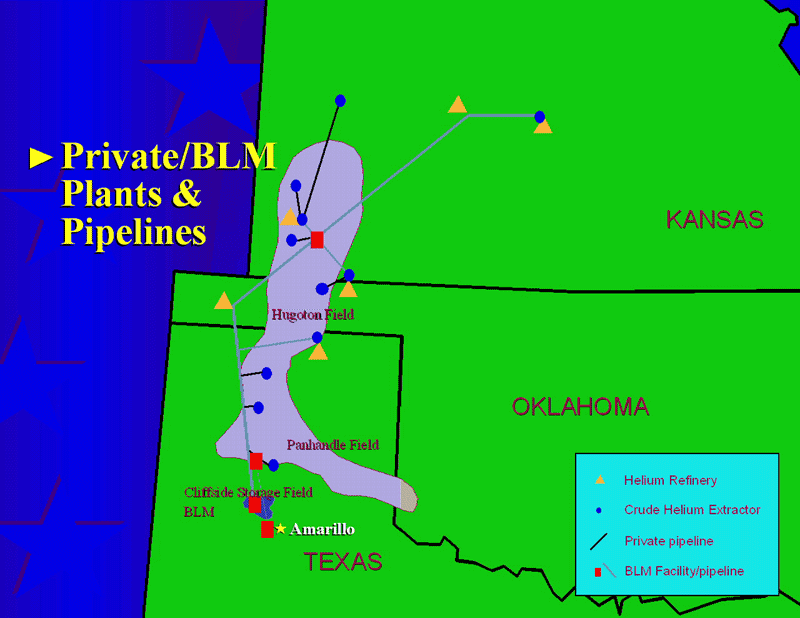
But we don’t replenish this Helium! We can mine and extract it, but once we take out what’s there, it will take either hundreds of millions of years to make more, or we need to find a new source of Helium, such as through mastering terrestrial nuclear fusion or perhaps mining the Moon.
In the meantime, we should be aware that every time we fill a Helium balloon, we’re taking something that it took the entire natural history of the Earth to create and sending it into the atmosphere, where the vast majority of it will wind up getting banished from our planet into the interstellar reaches of space.

Helium is a rare and unique element, with some amazing scientific properties. It doesn’t become a liquid until it’s cooled to a temperature of 4 Kelvin, and it becomes a superfluid with some fantastic, frictionless properties when it’s maybe half that temperature.
Helium is also used to supercool the world’s most powerful particle accelerators (such as the LHC), and is instrumental in the creation of the most powerful magnetic fields ever achieved on Earth.
As long as we don’t conserve and recycle our Helium, we are dooming mankind to a future shortage of Helium, and condemning future humans to figure out a way to effectively mine this trace element from the atmosphere, of which it makes up just 5 parts-per-million. Each balloon we fill with it (or inhale, for you teenagers) means there’s that much less Helium for future generations here on Earth. We only discovered this element less than 150 years ago, and the U.S. National Helium Reserve is expected to be empty by 2018, with the remainder of the Helium supply completely privatized.
I’m not here to scare you into not using Helium balloons or to convince you that we need government regulation of Helium production or consumption; I’m simply telling you where Helium comes from, and why we’re going to run out of it if we keep doing things the way we’re doing them. I’ve done my part by telling you the science behind our Helium; the rest is up to you.
An earlier version of this post originally appeared on the old Starts With A Bang blog at Scienceblogs.