Why Does Blowing On Your Hot Drink Cool It Down?

The Surprising Scientific Answer Has A Lot More Than Just Heat Exchange Involved.
“I like cappuccino, actually. But even a bad cup of coffee is better than no coffee at all.” –David Lynch
When it’s cold outside, it’s only natural to want something to warm you up. Whether that’s coffee, hot chocolate, tea, soup or another tasty beverage, it often comes to you too hot to actually put in your mouth, with the potential to scald you if you accidentally drink it right away. We all have our favorite ways to cool it down, though they all come with their disadvantages:
- You can simply wait for the cooler room to exchange energy with the hot liquid, but that often takes more time than most of us are willing to wait.
- You can drop an ice cube in there and speed up the cool-down process, but that waters down your beverage, something no one really wants.
- Or, you can blow on it, imparting your cool breath to the hot liquid, cooling it more quickly than leaving it alone without watering it down.
Almost all of us go with that last option by default, but the coolness of your breath is only part of the scientific story.

What is it that actually determines the temperature of your liquid? It’s how quickly the individual molecules inside are moving: their kinetic energy is a measure of their thermal energy. This is why, if you drop food coloring into both hot and cold water, they disperse in the hot water much faster than the cold: the molecules in the hot water move around much more quickly, and so, therefore, do the molecules of the food coloring, which quickly reach the same temperature as the water after being dropped in.
But if you looked closely at each individual molecule, you’d notice something a little more subtle at their speeds and their energies. Sure, after even a brief amount of time, they move at the same average speed and have the same average energy: this is the process of thermalization, where when molecules collide with one another, they exchange energy. Depending on the exact speeds, angles and masses of the colliding molecules, each individual molecule might be moving faster or slower than the average speed. In general, you get a certain type of distribution-of-speeds for the molecules inside your liquid: a Maxwell-Boltzmann distribution.
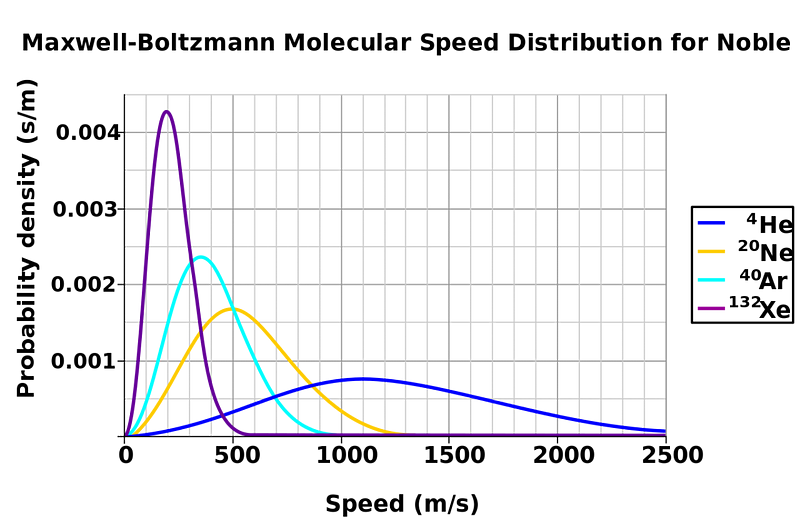
Sure, most of the molecules inside will have a speed that’s given by the average temperature, but there’s going to be a range: many will be hotter and many will be colder. What you don’t see on the diagram above is that for any temperature, some of the molecules (more for hotter temperatures, fewer for lower temperatures) will be above the temperature threshold to enter the gaseous phase. If you’ve ever observed steam rising off your hot beverage, that’s actually arising from the hottest, most energetic molecules inside entering the gaseous phase, condensing back into rising liquid droplets as the cool air above interacts with them. (Which is why, if you put your nose above a steaming cup of coffee, it comes out not just hot, but also wet!)

When you blow into the hot liquid, yes, the air you’re causing to come into contact with the liquid is cooler than the liquid itself, and so that heat exchange will help your beverage cool faster. But a big effect also comes from the fact that when you blow on your beverage, you’re increasing the number (and changing the sample) of molecules in contact with the air, and so you increase the rate at which the hottest molecules evaporate, entering the gaseous phase and leaving your liquid. The big reason this is important is when you allow the hottest molecules to escape, they take that heat with them, leaving you with an overall cooler system than you started with!
The next time someone berates you for blowing too strongly on your hot beverage, you’ll have science to back you up. A more vigorous blow helps the hottest molecules escape faster, leading you to enjoy your drink at the right temperature faster than anyone else!
Leave your comments on our forum, and check out our first book: Beyond The Galaxy, available now, as well as our reward-rich Patreon campaign!