A magnetic therapy for depression gains precision
In the mid-1970s, a British researcher named Anthony Barker wanted to measure the speed at which electrical signals travel down the long, slender nerves that can carry signals from the brain to muscles like those in the hand, triggering movement. To find out, he needed a way to stimulate nerves in people.
Researchers had already used electrodes placed on the skin to generate a magnetic field that penetrated human tissue — this produced an electric current that activated the peripheral nerves in the limbs. But the technique was painful, burning the skin. Barker, at the University of Sheffield in England, and his colleagues started to work on a better method.
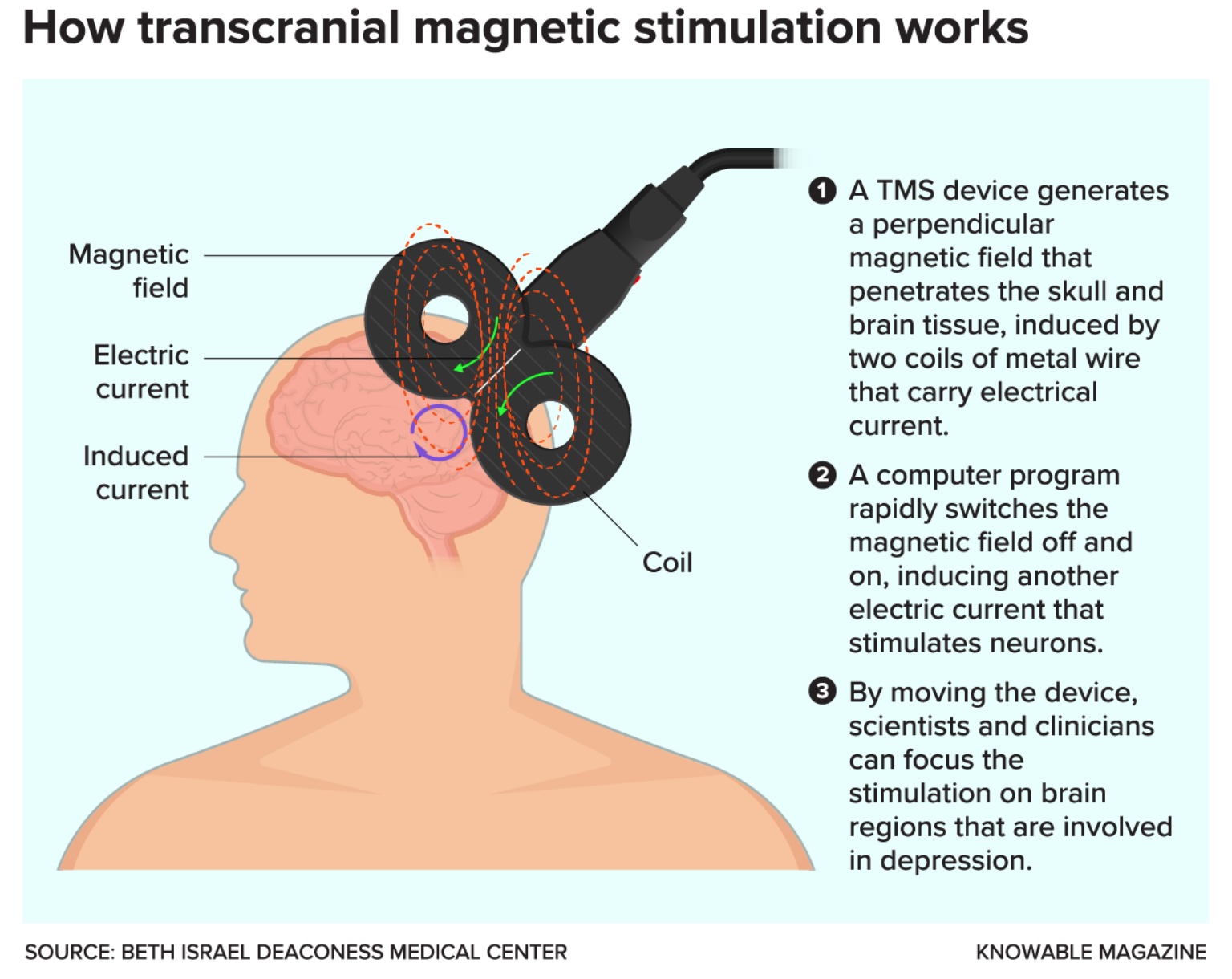
In 1985, with promising results under their belts, they tried positioning the coil-shaped magnetic device they’d developed on participants’ heads. The coil emitted rapidly alternating magnetic pulses over the brain region that controls movement, generating weak electrical currents in the brain tissue and activating neurons that control muscles in the hand. After about 20 milliseconds, the participants’ fingers twitched.
The technique, now called transcranial magnetic stimulation (TMS), has proved a vital tool for investigating how the human brain works. When targeted to specific brain regions, TMS can temporarily inhibit or enhance various functions – blocking the ability to speak, for instance, or making it easier to commit a series of numbers to memory. And when brain imaging technologies such as functional magnetic resonance imaging (fMRI) emerged in the 1990s, researchers could now “see” inside people’s brains as they received TMS stimulation. They could also observe how neural pathways respond differently to stimulation in psychiatric illnesses like schizophrenia and depression.
In recent decades, this fundamental research has yielded new treatments that alter brain activity, with TMS therapies for depression at the fore. In 2008, the US Food and Drug Administration approved NeuroStar, the nation’s first TMS depression device, and many other countries have since sanctioned the approach.
Yet even though TMS is now a widely available depression treatment, many questions remain about the method. It’s not clear how long the benefits of TMS can last, for example, or why it appears to work for some people with depression but not others. Another challenge is disentangling the effects of TMS from the placebo effect — when someone believes that they will benefit from treatment and gets better even though they’re receiving a “sham” form of stimulation.
Whether TMS can “cure” depression “is an open question — there’s evidence for and against,” says David Pitcher, a cognitive neuroscientist at the University of York who wrote a 2021 overview in the Annual Review of Psychology on using TMS to study human cognition. But as researchers fine-tune the approach and run more sophisticated clinical trials, TMS is emerging as a powerful tool for dissecting depression’s complexities — and for some people, loosening its grip.
From electric fish to magnets
TMS may be relatively new, but using electricity as medicine is ancient. As early as the 1st century CE, Roman physicians recommended using live electric torpedo fish to treat headache. Almost two millennia later, in the 1930s, physicians discovered that inducing brain seizures with electricity could reduce symptoms of schizophrenia and other forms of mental illness.
That was the beginning of electroconvulsive therapy (ECT) — pejoratively known as shock therapy. The practice spread rapidly despite the risk of memory loss, confusion and injuries from muscle spasms. Administering ECT without fully informing patients about the therapy and its risks also raised ethical concerns, an issue that medicine as a whole was seriously grappling with at the time.
Eventually, muscle relaxants, anesthetics and more stringent consent protocols improved ECT, although side effects such as headache and temporary short-term memory loss are still reported. ECT remains one of the most effective treatments for people who don’t respond to first-line antidepressant treatments such as serotonin reuptake inhibitors (SSRIs), a group of drugs that includes Zoloft and Prozac.
Yet both those drugs and ECT are difficult to control precisely, because they affect the whole brain. A more targeted approach is deep brain stimulation (DBS), which involves directly stimulating neurons with electrodes that are surgically implanted in regions known to affect mood and motivation. DBS has shown promise in pilot studies and was approved for investigative use in 2022, but has yet to receive clinical approval.
TMS, by contrast, requires no surgery and has fewer side effects than ECT, says Alvaro Pascual-Leone, a neurologist at Harvard Medical School. Though it’s not as easy to target to a specific brain region as DBS, it is much more precise than antidepressant drugs or ECT.
Pascual-Leone began investigating magnetic stimulation to treat major depressive disorder in the early 1990s. It excited him because of the ability to noninvasively focus the stimulation, and its few and relatively minor side effects, which commonly include scalp discomfort, tingling, spasms and lightheadedness and, rarely, seizures and hearing loss.
Although its mechanisms aren’t fully understood, TMS appears to act by reconfiguring neural circuits, kickstarting more typical communication between different brain areas, says Noah S. Philip, a psychiatrist at Brown University who researches TMS for treating depression.
At rest, people with depression often have reduced activity levels in an area of the brain called the dorsolateral prefrontal cortex (DLPFC) compared with non-depressed individuals, neuroimaging studies have shown. The region is a primary site for TMS therapy, says Philip. A hub for short-term memory, planning and abstract reasoning, the DLPFC is connected to several brain circuits implicated in depression: the salience network, which helps to focus attention on some things and ignore others; the default mode network, which is active when a person is not engaged in any particular task; and the executive network, key to planning, decision-making and impulse control. Together, these circuits underlie our ability to focus on relevant information and switch our attention between self-directed thinking and our environment.
Scientists think that abnormal communication between the networks can lead to the constant rumination, self-criticism and tendency to overly focus on negative aspects in life that people with depression often experience. Using fMRI to measure how nodes in the networks communicate before and after TMS, Philip, among other researchers, has found that multiple sessions of TMS can restore more typical activity. He says the change can endure beyond a year and can possibly be maintained longer with additional TMS treatments.
The sustained effects of TMS are likely due to the remodeling of neuronal connections brought on by microscopic changes to brain cells, Philip says. Studies have shown that TMS can stimulate neurons to sprout new dendrites, the branched appendages that receive signals from other neurons.
An early clinical trial found that people with severe depression who received daily TMS for several weeks were up to four times as likely to go into remission as participants in control groups, who were fitted with a TMS device but didn’t receive actual stimulation. In later studies that lacked control groups — meaning both the patients and the doctors knew TMS was being administered — 30 percent to 40 percent of patients who hadn’t improved with medications went into remission, defined by a low score on a standard measure such as the Hamilton Depression Rating Scale. That’s roughly on par with the number of people who respond well to antidepressant medications, according to a 2013 review in Current Opinion in Psychiatry.
Still, many questions remain — including whether better results can be gained by targeting specific regions in individual patients. “Some people with depression experience sadness, others have more of a lack of motivation or apathy,” says Pascual-Leone. “Some people eat too much; others eat too little.” Unlike more general treatments, he says, TMS holds such targeting potential.
Bespoke brain stimulation
In many clinics and research labs, the process of determining where to place TMS coils on a person’s head is relatively simple. The FDA-sanctioned method harks back to Barker’s original experiments: A participant sticks their thumb out like a hitchhiker, and technicians move the magnetic coil around their scalp until the electrical stimulation hits a part of the motor cortex that makes their thumb involuntarily twitch. Using this spot as their landmark, the technicians then move the coil to a position that targets the left DLPFC.
The approach is moderately effective, but many researchers think it doesn’t adequately account for the large variations in brain structure among individuals. Increasingly, scientists are using fMRI and other brain imaging technologies to tailor TMS stimulation to each person’s unique brain structure and observe how it affects their neural activity patterns. “An exciting advance in the last 10 years with TMS is to use it on patients, then neuroimage their brain to look at changes in the connectivity between the DLPFC and the areas we know it’s connected to,” says Pitcher of the University of York.
A team of investigators at Stanford University, led by psychiatrist Nolan Williams, director of the Stanford Brain Stimulation Lab, is one of the groups developing this combined approach. In a small 2021 study, the team used fMRI scans, which measure changes in blood flow associated with brain activity, to locate a small subregion in the DLPFC in individual patients. This subregion’s activity shows an inverse relationship to that of another brain area, the subgenual cingulate (SGC): In people who are experiencing depression, the SGC’s activity is boosted while the DLPFC subregion’s activity is lowered. Conversely, the more active the node in the DLPFC, the less active the SGC becomes. The SGC, for its part, appears to influence the default mode network, anchoring people in negative patterns of self-rumination.
In the Stanford study, Williams and colleagues targeted the DLPFC subregion in 29 people with what’s known as treatment-resistant depression: They scored “moderate to severe” on a standard assessment that considers how they responded to previous depression treatments and the duration and severity of their symptoms. The group received an experimental, accelerated treatment regimen with more than one daily TMS session. To control for a potential placebo effect, some group members were randomly assigned to receive sham stimulation that sounded and felt like TMS but didn’t deliver the electromagnetic pulses.
After five days of treatment, 79 percent of the participants who received the focused TMS experienced remission, compared with 13 percent of the control group. The team also observed that stimulating the area of the DLPFC that’s most strongly connected with the SGC normalizes the relevant connections among the three regions. “You see it when you scan people after,” Williams says.
One participant in his early sixties, Tommy Van Brocklin, had battled depression for about 45 years. In recent years, the medication he had been taking stopped working.
The five-day treatment made him feel “like a tree being pecked by a woodpecker,” because of the knocking sounds that the TMS device emits, he jokes. But on day three, he noticed a difference in his mood: “Everything seemed to kind of kick into gear, and it was good.”
The researchers believe this individualized approach to TMS treatment could be a fast, effective intervention for suicidal patients. In 2022, Williams’s method passed an important regulatory hurdle: The FDA granted permission to commercialize it.
Seeing such high remission rates after just five days of treatment — a highly practical intervention — is exciting, says William T. Regenold, director of a research unit that studies noninvasive neuromodulation at the US National Institute of Mental Health. He and his colleagues are currently conducting a clinical trial investigating changes in brain activity in severely depressed patients who receive TMS along with talk therapy. “The idea is to have a synergistic effect between the psychotherapy and the TMS,” he says.
By adjusting the treatment to each person’s brain anatomy, the Stanford study’s success is “very aligned with the notions of precision medicine — of individually targeted interventions,” says Pascual-Leone.
But there’s still much work ahead. One urgent challenge is to tease out the benefits of stimulation from the placebo effect; some research suggests that this effect plays a role in the improvements that many experience from TMS. This problem isn’t unique to brain stimulation, but is common to all depression treatments. Several meta-analyses of the placebo effect for antidepressant medications, for example, have found that people who get inactive pills experience a 20 percent to 40 percent improvement in their symptoms, typically measured by one of several standard questionnaires.
Research groups are starting to achieve clearer, more impressive outcomes using brain scans to guide individualized stimulation, as the Stanford team did. But they’ll need more studies to determine if TMS will find similar success in larger populations, as well as in groups such as teenagers, the elderly and people who have conditions that often accompany depression, such as anxiety and post-traumatic stress. Some labs are experimenting with the design of TMS devices — comparing figure-8-shaped magnetic coils to ones shaped like butterfly wings, for example — and altering the frequency of stimulation to see how that alters brain activity and treatment outcomes.
Still, many fundamental questions about TMS remain. Although scientists know it disrupts normal neuronal firing, “we don’t really understand how TMS works in a mechanistic way” to alter brain states like mood, says Pitcher. One reason is that researchers can’t record activity from a single neuron in a human being and do TMS at the same time, he says. It’s not clear why certain frequencies of TMS appear to ramp up activity in some brain regions, while turning it down in other areas — or how the new neuronal connections spurred by TMS affect the different brain networks.
“Fortunately, we can do that work, advancing science while helping people with disabling diseases,” says Pascual-Leone. “That’s an amazing position to be in.”
This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Sign up for Knowable Magazine’s newsletter.