Does light itself truly have an infinite lifetime?

- In the expanding Universe, for billions upon billions of years, the photon seems to be one of the very few particles that has an apparently infinite lifetime.
- Photons are the quanta that compose light, and in the absence of any other interactions that force them to change their properties, are eternally stable, with no hint that they would transmute into any other particle.
- But how well do we know this to be true, and what evidence can we point to in order to determine their stability? It’s a fascinating question that pushes us right to the limits of what we can scientifically observe and measure.
One of the most enduring ideas in all the Universe is that everything that exists now will someday see its existence come to an end. The stars, galaxies, and even the black holes that occupy the space in our Universe will all some day burn out, fade away, and otherwise decay, leaving what we think of as a “heat death” state: where no more energy can possibly be extracted, in any way, from a uniform, maximum entropy, equilibrium state. But, perhaps, there are exceptions to this general rule, and that some things will truly live on forever.
One such candidate for a truly stable entity is the photon: the quantum of light. All of the electromagnetic radiation that exists in the Universe is made up of photons, and photons, as far as we can tell, have an infinite lifetime. Does that mean that light will truly live forever? That’s what Anna-Maria Galante wants to know, writing in to ask:
“Do photons live forever? Or do they ‘die,’ and convert to some other particle? The light we see erupting from cosmic events over a verrrrry long past … we seem to know where it comes from, but where does it go? What is the life cycle of a photon?”
It’s a big and compelling question, and one that brings us right up to the edge of everything we know about the Universe. Here’s the best answer that science has today.

The first time the question of a photon having a finite lifetime came up, it was for a very good reason: we had just discovered the key evidence for the expanding Universe. The spiral and elliptical nebulae in the sky were shown to be galaxies, or “island Universes” as they were then known, well beyond the scale and scope of the Milky Way. These collections of millions, billions, or even trillions of stars were located at least millions of light-years away, placing them well outside of the Milky Way. Moreover, it was quickly shown that these distant objects weren’t just far away, but they appeared to be receding from us, as the more distant they were, on average, the greater the light from them turned out to be systematically shifted toward redder and redder wavelengths.
Of course, by the time this data was widely available in the 1920s and 1930s, we had already learned about the quantum nature of light, which taught us that the wavelength of light determined its energy. We also had both special and general relativity well in hand, which taught us that once the light leaves its source, the only way you could change its frequency was to either:
- have it interact with some form of matter and/or energy,
- have the observer moving either toward or away from the observer,
- or to have the curvature properties of space itself change, such as due to a gravitational redshift/blueshift or an expansion/contraction of the Universe.
The first potential explanation, in particular, led to the formulation of a fascinating alternative cosmology: tired light cosmology.

First formulated in 1929 by Fritz Zwicky — yes, the same Fritz Zwicky who coined the term supernova, who first formulated the dark matter hypothesis, and who once tried to “still” the turbulent atmospheric air by firing a rifle through his telescope tube — the tired light hypothesis put forth the notion that propagating light loses energy through collisions with other particles present in the space between galaxies. The more space there was to propagate through, the logic went, the more energy would be lost to these interactions, and that would be the explanation, rather than peculiar velocities or cosmic expansion, for why light appeared to be more severely redshifted for more distant objects.
However, in order for this scenario to be correct, there are two predictions that should be true.
1. ) When light travels through a medium, even a sparse medium, it slows down from the speed of light in vacuum to the speed of light in that medium. The slowdown affects light of different frequencies by different amounts. Just as light passing through a prism splits into different colors, light passing through an intergalactic medium that interacted with it should slow light of different wavelengths down by different amounts. When that light re-enters a true vacuum, it will resume moving at the speed of light in a vacuum.
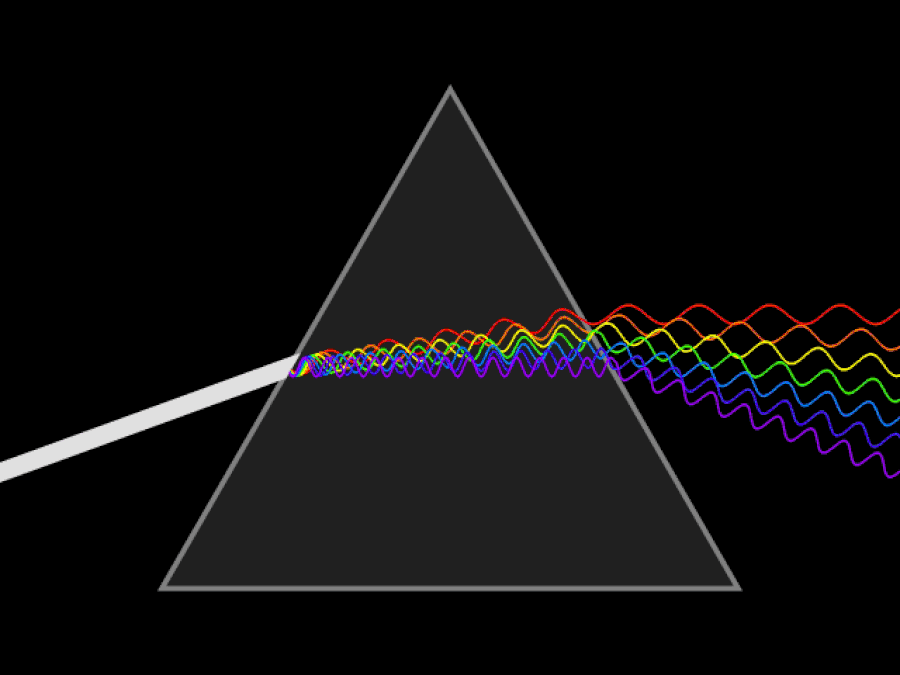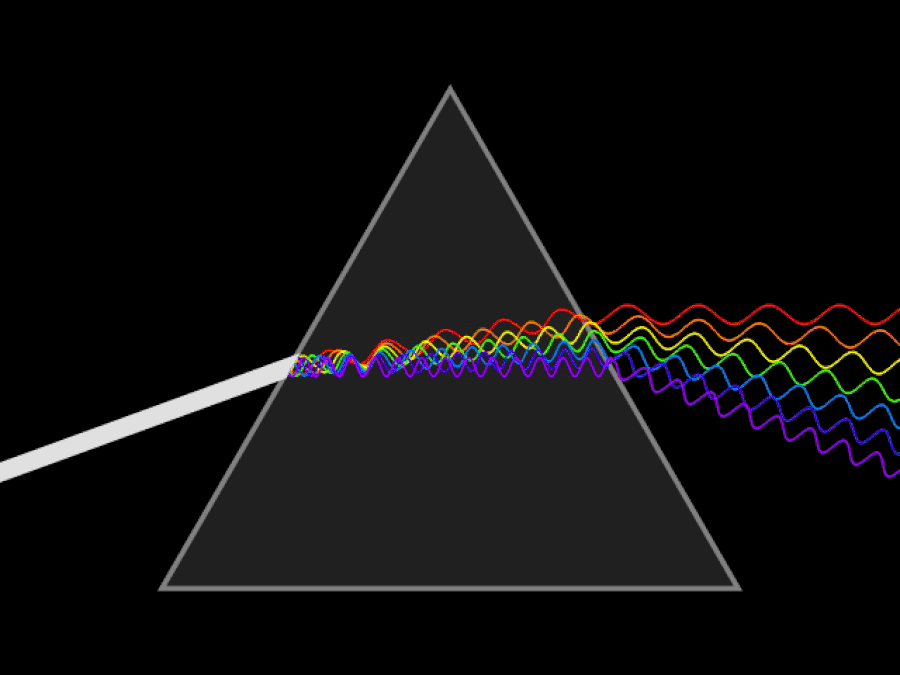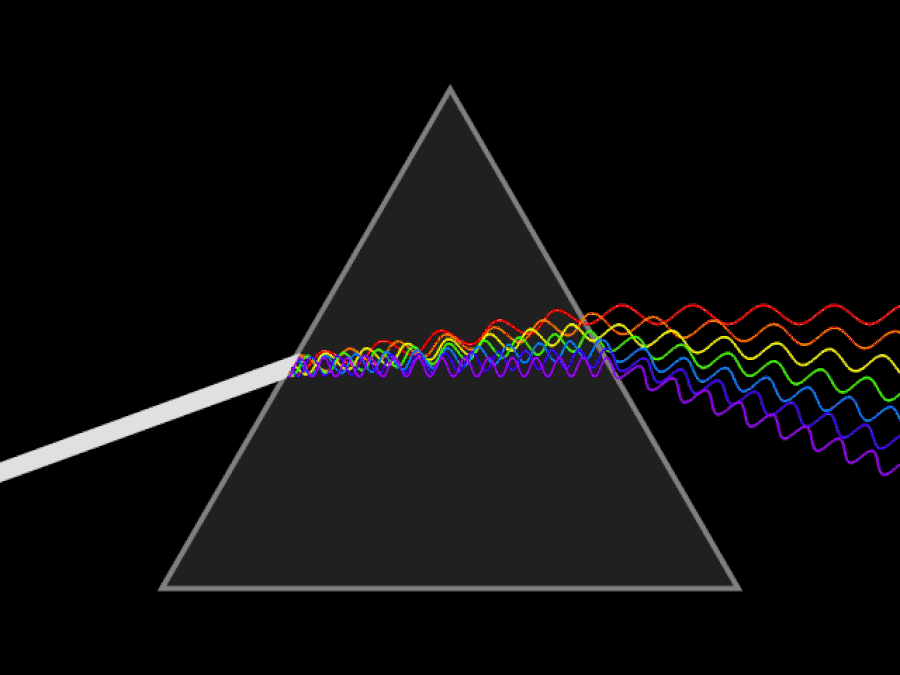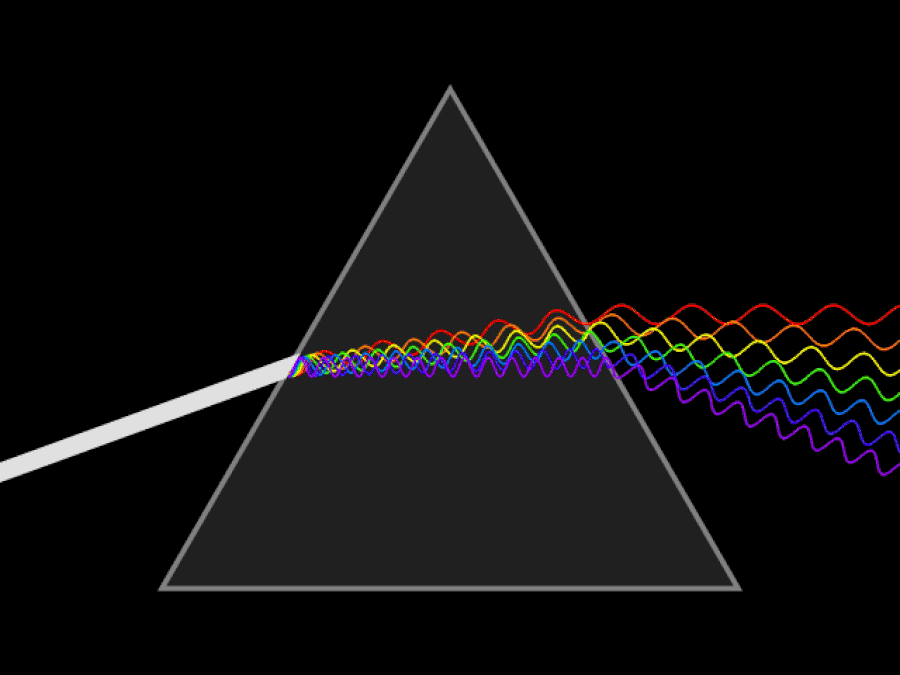
And yet, when we observed the light coming from sources at different distances, we found no wavelength-dependence to the amount of redshift that light exhibited. Instead, at all distances, all wavelengths of emitted light are observed to redshift by the exact same factor as all others; there is no wavelength-dependence to the redshift. Because of this null observation, the first prediction of tired light cosmology is falsified.
But there’s a second prediction to contend with, as well.
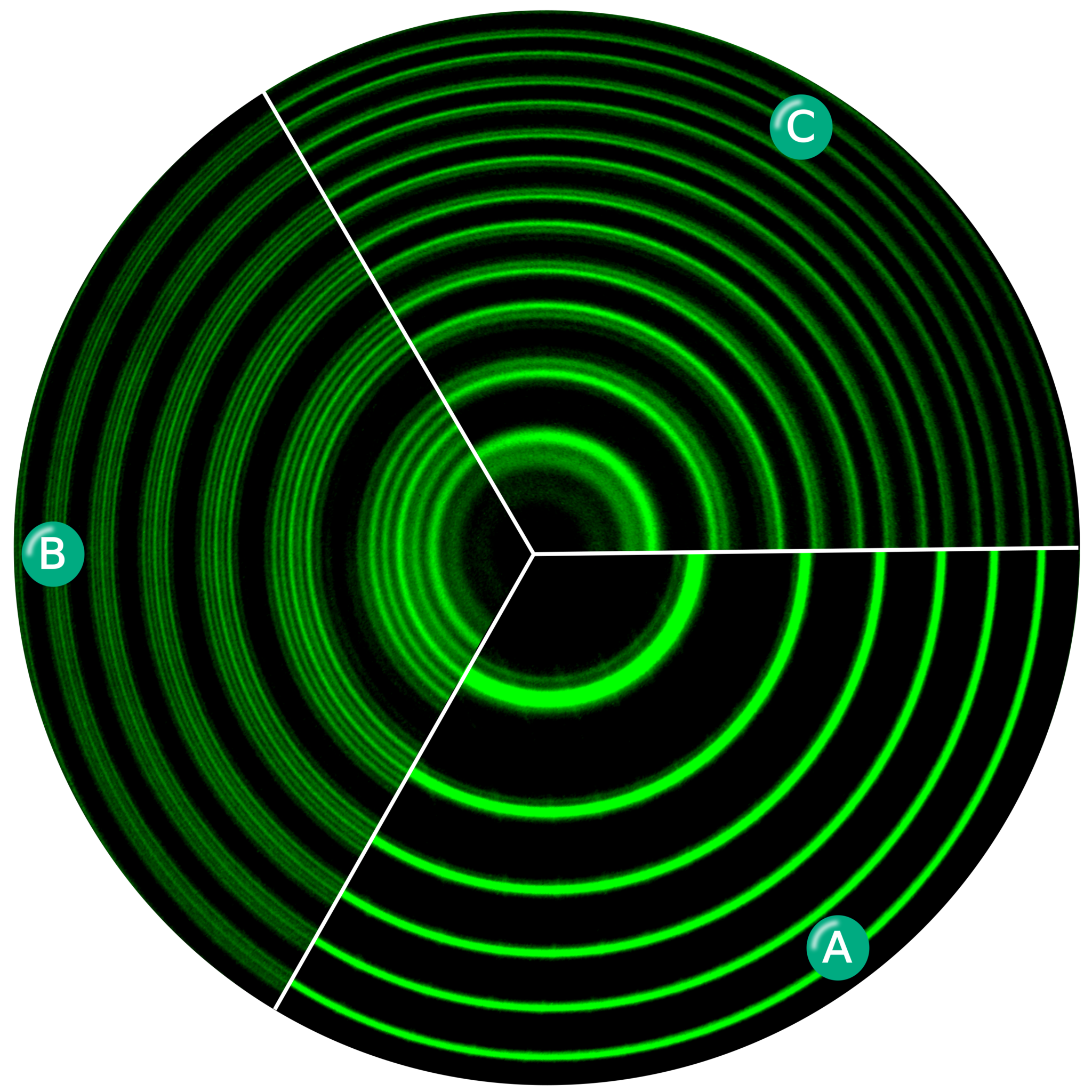
2.) If more distant light loses more energy by passing through a greater length of a “lossy medium” than less distant light, then those more distant objects should appear to be blurred by a progressively greater and greater amount than the less distant ones.
And again, when we go to test this prediction, we find that it isn’t borne out by observations at all. More distant galaxies, when seen alongside less distant galaxies, appear just as sharp and high-resolution as the less distant ones. This is true, for example, for all five of the galaxies in Stephan’s Quintet, as well as for the background galaxies visible behind all five of the quintet’s members. This prediction is falsified as well.

While these observations are good enough to falsify the tired light hypothesis — and, in fact, were good enough to falsify it immediately, as soon as it was proposed — that’s only one possible way that light could be unstable. Light could either die out or convert into some other particle, and there’s a set of interesting ways to think about these possibilities.
The first arises simply from the fact that we have a cosmological redshift. Each and every photon that’s produced, irrespective of how it’s produced, whether thermally or from a quantum transition or from any other interaction, will stream through the Universe until it collides and interacts with another quantum of energy. But if you were a photon emitted from a quantum transition, unless you can engage in the inverse quantum reaction in rather rapid fashion, you’re going to begin traveling through intergalactic space, with your wavelength stretching due to the Universe’s expansion as you do. If you’re not lucky enough to be absorbed by a quantum bound state with the right allowable transition frequency, you’ll simply redshift and redshift until you’re below the longest possible wavelength that will ever allow you to be absorbed by such a transition ever again.
However, there’s a second set of possibilities that exists for all photons: they can interact with an otherwise free quantum particle, producing one of any number of effects.
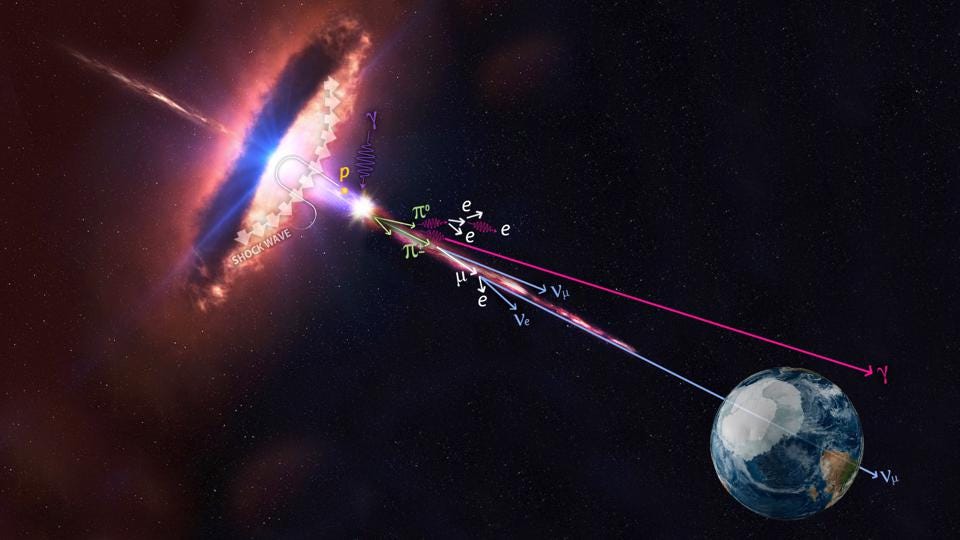
This can include scattering, where a charged particle — usually an electron — absorbs and then re-emits a photon. This involves an exchange of both energy and momentum, and can boost either the charged particle or the photon to higher energies, at the expense of leaving the other one with less energy.
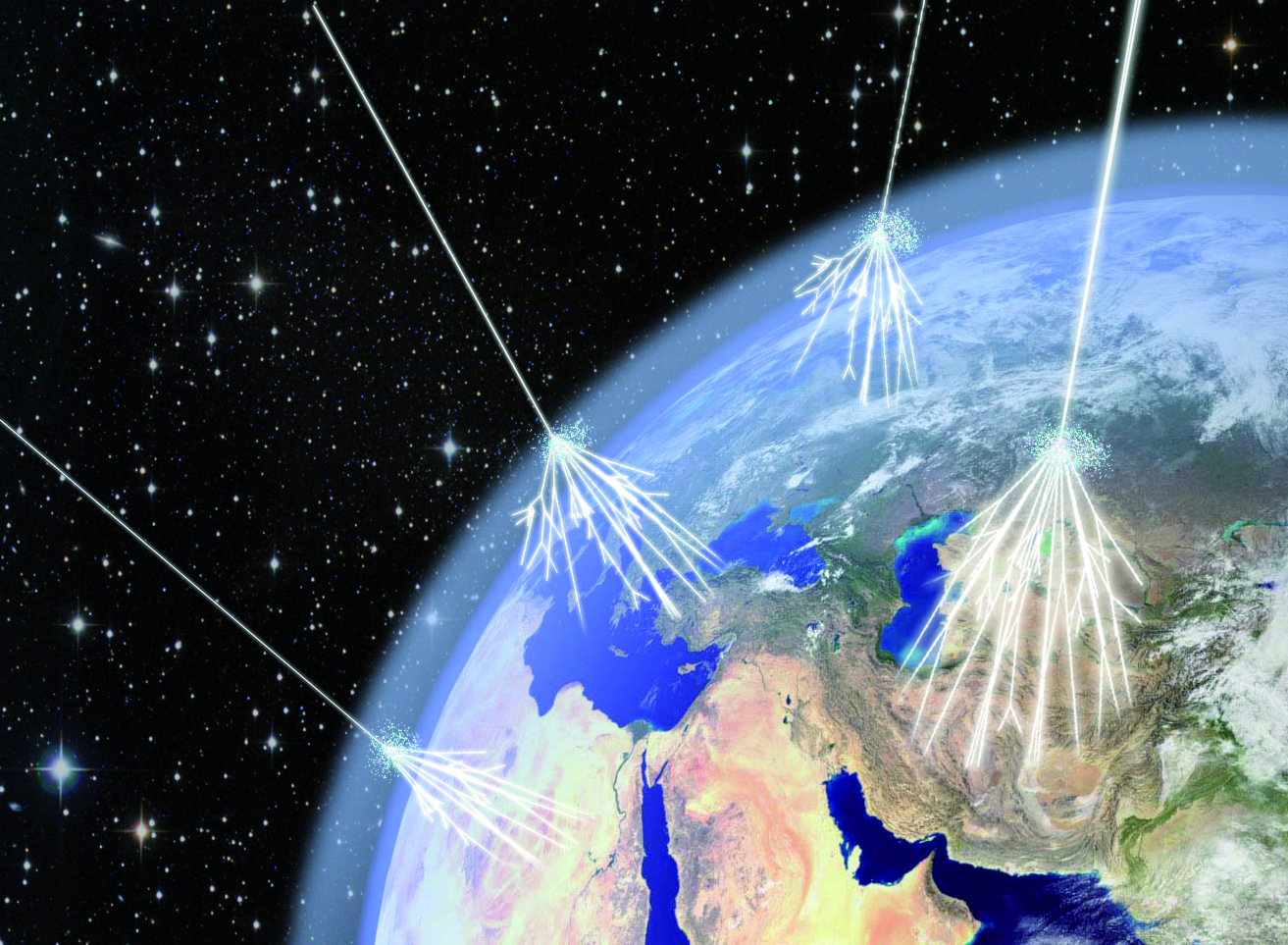
At high enough energies, the collision of a photon with another particle — even another photon, if the energy is high enough — can spontaneously produce a particle-antiparticle pair if there’s enough available energy to make them both through Einstein’s E = mc². In fact, the highest energy cosmic rays of all can do this even with the remarkably low-energy photons that are a part of the cosmic microwave background: the Big Bang’s leftover glow. For cosmic rays above ~1017 eV in energy, a single, typical CMB photon has a chance to produce electron-positron pairs. At even higher energies, more like ~1020 eV in energy, a CMB photon has a significantly large chance to convert to a neutral pion, which robs cosmic rays of energy rather quickly. This is the primary reason why there’s a steep drop-off in the population of the highest-energy cosmic rays: they’re above this critical energy threshold.
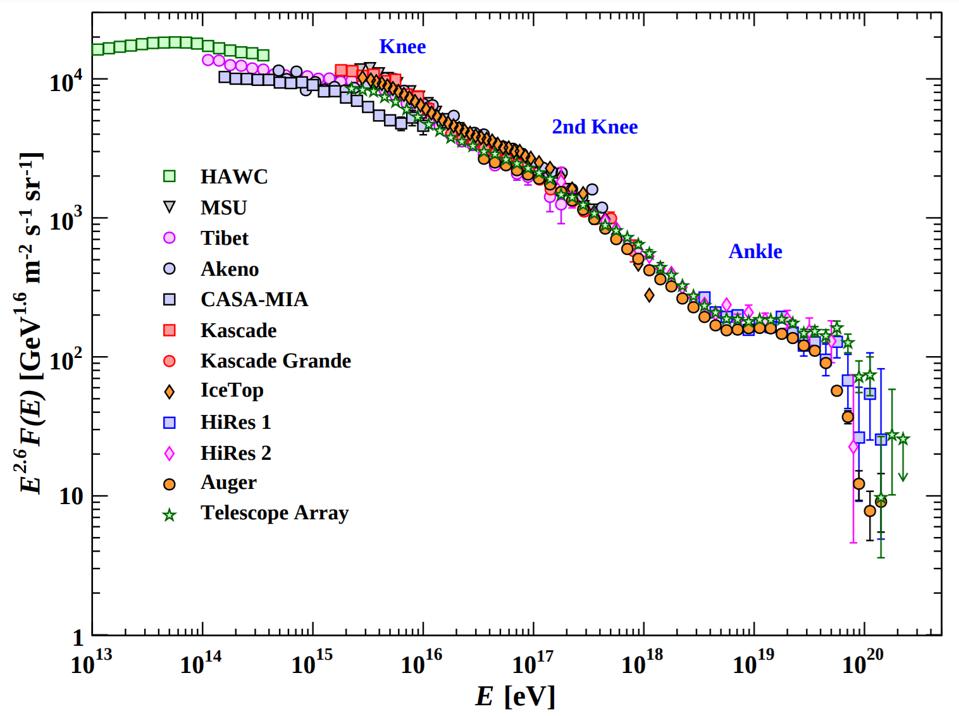
In other words, even very low-energy photons can be converted into other particles — non-photons — by colliding with another high-enough-energy particle.
There’s yet a third way to alter a photon beyond cosmic expansion or through converting into particles with a non-zero rest mass: by scattering off of a particle that results in the production of still additional photons. In practically every electromagnetic interaction, or interaction between a charged particle and at least one photon, there are what are known as “radiative corrections” that arise in quantum field theories. For every standard interaction where the same number of photons exist at the beginning as at the end, there’s a little less than a 1% chance — more like 1/137, to be specific — that you’ll wind up radiating an additional photon in the end over the number you started off with.
And every time you have an energetic particle that possesses a positive rest mass and a positive temperature, those particles will also radiate photons away: losing energy in the form of photons.
Photons are very, very easy to create, and while it’s possible to absorb them by inducing the proper quantum transitions, most excitations will de-excite after a given amount of time. Just like the old saying that “What goes up must come down,” quantum systems that get excited to higher energies through the absorption of photons will eventually de-excite as well, producing at least the same number of photons, generally with the same net energy, as were absorbed in the first place.
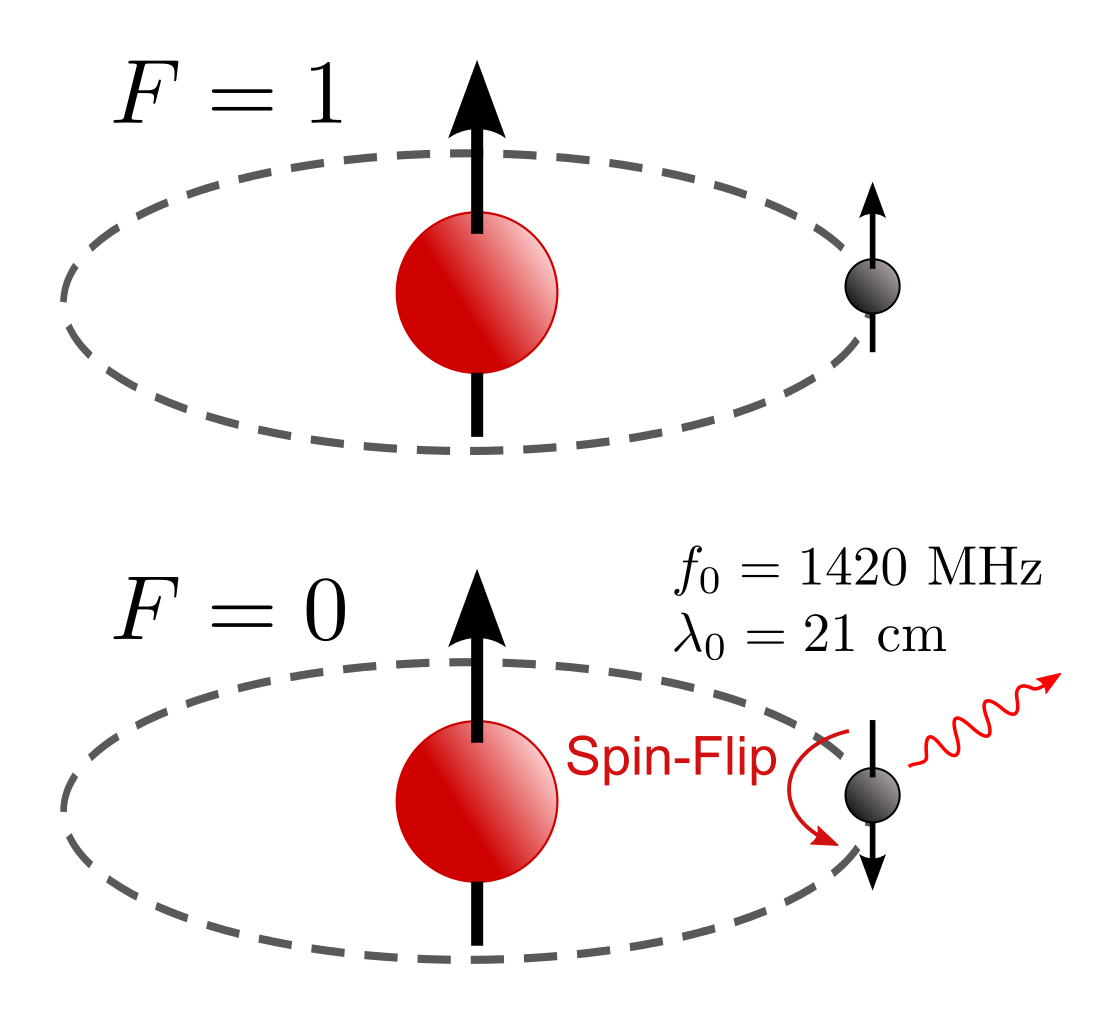
Given that there are so many ways to create photons, you’re probably salivating for ways to destroy them. After all, simply waiting for the effects of cosmic redshift to bring them down to an asymptotically low energy value and density is going to take an arbitrarily long time. Each time the Universe stretches to become larger by a factor of 2, the total energy density in the form of photons drops by a factor of 16: a factor of 24. A factor of 8 comes because the number of photons — despite all the ways there are to create them — remains relatively fixed, and doubling the distance between objects increases the volume of the observable Universe by a factor of 8: double the length, double the width, and double the depth.
The fourth-and-final factor of two comes from the cosmological expansion, which stretches the wavelength to double its original wavelength, thereby halving the energy-per-photon. On long enough timescales, this will cause the energy density of the Universe in the form of photons to asymptotically drop toward zero, but it will never quite reach it.

You might try to get clever, and imagine some sort of exotic, ultra-low-mass particle that couples to photons, that a photon could convert into under the right conditions. Some sort of boson or pseudoscalar particle — like an axion or axino, a neutrino condensate, or some sort of exotic Cooper pair — could lead to precisely this sort of occurrence, but again, this only works if the photon is sufficiently high in energy to convert to the particle with a non-zero rest mass via E = mc². Once the photon’s energy redshifts below a critical threshold, that no longer works.
Similarly, you might imagine the ultimate way to absorb photons: by having them encounter a black hole. Once anything crosses over from outside the event horizon to inside it, it not only can never escape, but it will always add to the rest mass energy of the black hole itself. Yes, there will be many black holes populating the Universe over time, and they will grow in mass and size as time continues forward.
But even that will only occur up to a point. Once the density of the Universe drops below a certain threshold, black holes will begin decaying via Hawking radiation faster than they grow, and that means the production of even greater numbers of photons than went into the black hole in the first place. Over the next ~10100 years or so, every black hole in the Universe will eventually decay away completely, with the overwhelming majority of the decay products being photons.

So will they ever die out? Not according to the currently understood laws of physics. In fact, the situation is even more dire than you probably realize. You can think of every photon that was or will be:
- created in the Big Bang,
- created from quantum transitions,
- created from radiative corrections,
- created through the emission of energy,
- or created via black hole decay,
and even if you wait for all of those photons to reach arbitrarily low energies due to the Universe’s expansion, the Universe still won’t be devoid of photons.
Why’s that?
Because the Universe still has dark energy in it. Just as an object with an event horizon, like a black hole, will continuously emit photons due to the difference in acceleration close to versus far away from the event horizon, so too will an object with a cosmological (or, more technically, a Rindler) horizon. Einstein’s equivalence principle tells us that observers cannot tell the difference between gravitational acceleration or acceleration due to any other cause, and any two unbound locations will appear to accelerate relative to one another owing to the presence of dark energy. The physics that results is identical: a continuous amount of thermal radiation gets emitted. Based on the value of the cosmological constant we infer today, that means a blackbody spectrum of radiation with a temperature of ~10–30 K will always permeate all of space, no matter how far into the future we go.
Even at its very end, no matter how far into the future we go, the Universe will always continue to produce radiation, ensuring that it will never reach absolute zero, that it will always contain photons, and that even at the lowest energies it will ever reach, there ought to be nothing else for the photon to decay or transition into. Although the energy density of the Universe will continue to drop as the Universe expands, and the energy inherent to any individual photon will continue to drop as time ticks onward and onward into the future, there will never be anything “more fundamental” that they transition into.
There are exotic scenarios we can cook up that will change the story, of course. Perhaps it’s possible that photons really do have a non-zero rest mass, causing them to slow down to slower than the speed of light when enough time passes. Perhaps photons really are inherently unstable, and there’s something else that’s truly massless, like a combination of gravitons, that they can decay into. And perhaps there’s some sort of phase transition that will occur, far into the future, where the photon will reveal its true instability and will decay into a yet-unknown quantum state.
But if all we have is the photon as we understand it in the Standard Model, then the photon is truly stable. A Universe filled with dark energy ensures, even as the photons that exist today redshift to arbitrarily low energies, that new ones will always get created, leading to a Universe with a finite and positive photon number and photon energy density at all times. We can only be certain of the rules to the extent that we’ve measured them, but unless there’s a big piece of the puzzle missing that we simply haven’t uncovered yet, we can count on the fact that photons might fade away, but they’ll never truly die.
Ethan is on medical leave until May 6th. Please enjoy a republication of this article from the Starts With A Bang archives!