Throwback Thursday: The Physics of Hot Pockets
From why they stay frozen in the middle to how the crisping sleeve works!
“Is your Hot Pocket cold in the middle?”
“It’s frozen. But it can be served boiling lava hot.”
“Will it burn my mouth?”
“It’ll destroy your mouth. Everything will taste like rubber for a month.”
-Jim Gaffigan
(Thanks to Michael Broide for the idea for this post, and a fantastic discussion of this phenomenon with me way back in 2009.) When I was a teenager, my choice of food was basically determined by three criteria:
- It needed to be ready in under 10 minutes.
- It needed to have enough calories to make me not hungry anymore.
- And it needed to be (marginally) edible.
That was really it. Basically, if you could serve me something that was hot with fat and salt in it, I wasn’t complaining. And so it won’t surprise you to know that a portion of my diet at the time consisted of — you guessed it — Hot Pockets. And to be fair, they don’t actually look so bad on the box.

But if you’ve ever tried to microwave one, even using the vaunted “crisping sleeve” that comes with it, you’ve very likely run into the same problem that Jim Gaffigan so eloquently described: if you cook it for the recommended time, the very outer layer gets crispy, the outer edges of the interior come out boiling lava hot, and the middle remains completely frozen.
Your only alternative is to nuke the everliving daylights out of it, in which case the entire thing will not only give you second degree burns, but will explode inside your microwave.

So why does this happen? Let’s back up and take you to a place where this doesn’t happen: inside your conventional oven. When you turn on your oven, the heating element clicks on, the air inside heats up to a uniform temperature, the heating element clicks off, and then you put your food inside.
Over time, the air will cool, the heating element will turn back on again, heating the air back up to the temperature you set it to, and then go back off. The hot surrounding air is what’s cooking or reheating your food, and it’s doing it slowly, gradually, from the outside-in. Because of how heat transport works, this method of cooking (or reheating) is slow, but relatively uniform.

But not everybody has time for that. Instead, you can broil your food, which is how a toaster works, a toaster oven, or a conventional oven set to broil (or simply with the heating elements continuously on). In this case, you’ve got a very hot source that’s constant radiating infrared light (heat) onto your food, as the heating element microscopically vibrates back-and-forth.
This method gives a nice “sear” to your food, as the energy of the radiation hitting your food is much greater than in the air-cooking method that conventional ovens use. This method does have the advantage of crisping up whatever you’re cooking rather quickly, but you can also very easily burn the outside of what you’re cooking. And only in very rare cases is that desirable.

Which brings us to the microwave. Microwave ovens work by producing microwaves, which are just a type of electromagnetic radiation, much like visible light, infrared radiation (i.e., heat), and radio waves. But microwaves don’t heat your food directly; they’re special because they are just the right wavelength to be absorbed by three important classes of molecules: fats, sugars and water. And water molecules — in particular — do something special. As you know, water molecules are very simple, with an electronegative oxygen atom and two electropositive hydrogen atoms.
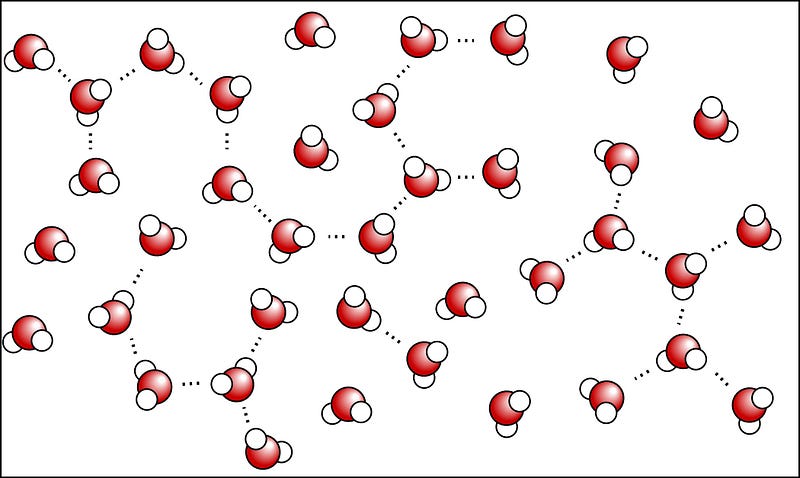
Normally, water molecules in the liquid state are loosely bound together via hydrogen bonds (shown as dots, above), where the relatively negatively-charged part of one molecule is attracted to the relatively positively-charged part of another. But if you bombard the water molecules with microwaves — which have their electric fields switch directions at just the right frequency to strongly influence the water molecules — the water molecules begin to rotate as a result.
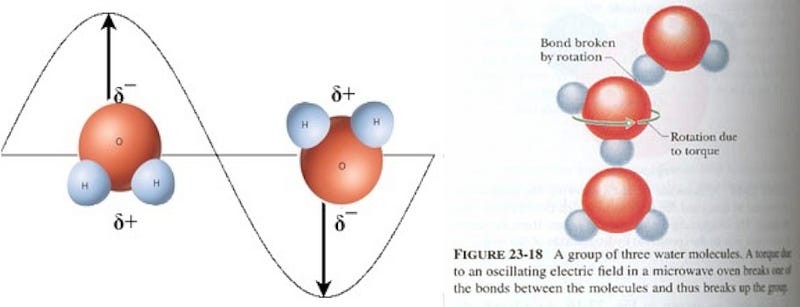
Which explains why microwaves are so good for heating up and quickly boiling liquid water: rotational motion is a form of energy, and that excess energy causes the water to heat up! But there’s a problem for your frozen Hot Pockets. You see, the water molecules in it aren’t free to move; they’re frozen in a crystal ice lattice!

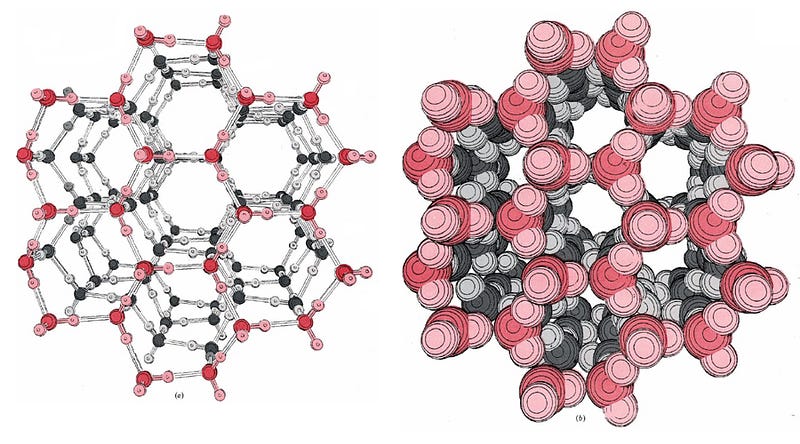
On a molecular level, the individual water molecules are arranged into hexagonal rings, where they’re rigidly held in place. These then form ice lattices of varied shapes dependent on a number of conditions, but in all cases, these bonds are very strong, and — most importantly — the individual water molecules in this state are not free to rotate! Even a changing electric field due to a powerful microwave isn’t going to break those water molecules out of their lattice.
Which means that microwaves don’t heat ice, not nearly as efficiently as they heat water! The outermost layer of your frozen food — as soon as you removed it from the freezer — has just been exposed to room-temperature air, so some of those crystals have started to melt. That outer, liquid-watery layer can heat up quickly. But all the layers inside are ice, and hence lousy at absorbing microwaves! So the outer layer has to get hot enough to melt the ice crystals interior in the next layer. Then that newly-melted water can heat up and melt the ice farther in.
In the meantime, however, the outermost layer gets really hot! As in, boiling lava hot. And that is why Hot Pockets can both destroy your mouth and be frozen on the inside at the same time!

But what about the crisping sleeve? That’s actually a true stroke of brilliance, and combines the best aspects of a generic broiler with a microwave! What you do is you take a thin film of metal that can absorb the electromagnetic (microwave) radiation and convert it to heat, where it re-radiates back out onto the food. This type of material in this particular use case is known as a susceptor, where susceptance is a metal’s ability to convert electromagnetic radiation into heat. A good susceptor can get very hot, and will work almost as well as a heating element inside a toaster oven!
In an ideal world, there would be a gap of air in between your susceptor and your food, allowing the metal to heat up to very high temperatures, where it can then “crisp up” your food. Because of the way hot pockets actually sit on the bottom of the sleeve, you wind up with three crispy sides and one decidedly less crispy one, a consequence of not having a gap between the food and the thin, metal film on the bottom!

And from end-to-end, microwaves rotating liquid (but not solid) water molecules and the susceptor-in-a-crisping-sleeve with an imperfect air gap, that’s the physics of hot pockets! (Or any frozen food.)
And for those of you who stuck with me this long, here’s your Jim Gaffigan reward! Haaaaahhhh-ot Pockets!
https://www.youtube.com/watch?v=wmHSe_S04CU
Enjoyed this? Leave a comment on the Starts With A Bang forum at Scienceblogs!