Water in Space: What Happens?
How one of the most interesting molecules on Earth behaves in the zero-gravity, zero-pressure environment of outer space.
“Day after day, day after day,
We stuck, nor breath nor motion;
As idle as a painted ship
Upon a painted ocean.
Water, water, every where,
And all the boards did shrink;
Water, water, every where,
Nor any drop to drink.” -Rime of the Ancient Mariner, Samuel Taylor Coleridge
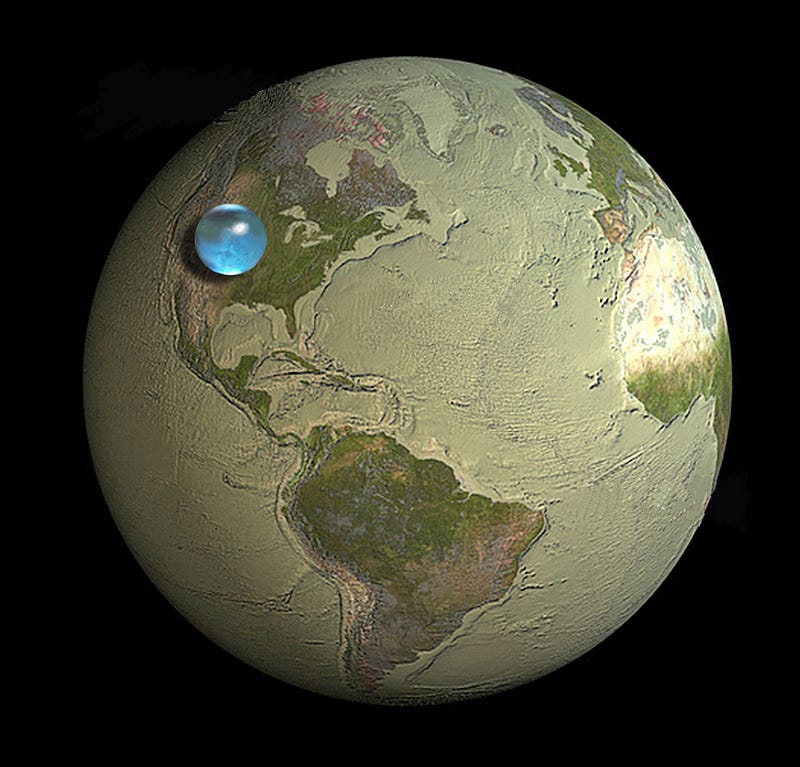
The Earth is one of those extremely rare, special places in the Universe where water can exist, stably, as a liquid. Our blue marble is so familiar to us that we forget how rare liquid water is in the Universe.

So much of it exists here on Earth, that if you were to add up all the oceans on Earth together, it would weigh more than 10^18 tonnes, more massive than the biggest asteroid ever, and about as massive as Pluto’s giant moon, Charon. All told, that’s a lot of water, enough to fill a sphere 1,385 km in diameter!
But water only has a very small window in which it can physically exist as a liquid, even here on Earth. For instance, if you took some warm water up to a very high elevation, it would start to boil, and become a gas! The higher up you took it, the lower and lower your boiling point would be.
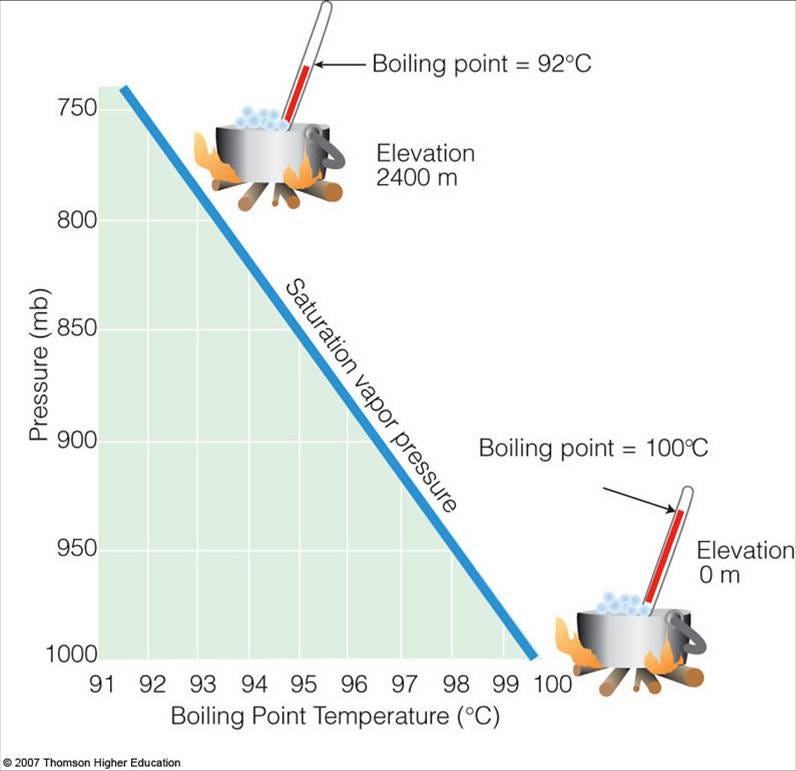
Why is that? Because higher altitudes on Earth have a smaller percentage of the atmosphere pushing down on top of it, meaning lower pressure. At normal atmospheric temperatures here on Earth, water molecules have a certain amount of kinetic energy, and tend to move with a certain average speed. A few of those molecules will have enough energy, at any given time, to escape the liquid phase and become a gas; the biggest force that counteracts this comes from the atmospheric pressure. Increase the pressure, and it becomes more difficult for water to escape as a gas; decrease the pressure and it becomes easier. That’s why the boiling temperature of water is higher inside a pressure cooker, but lower at high altitudes, where the atmospheric pressure is lower.
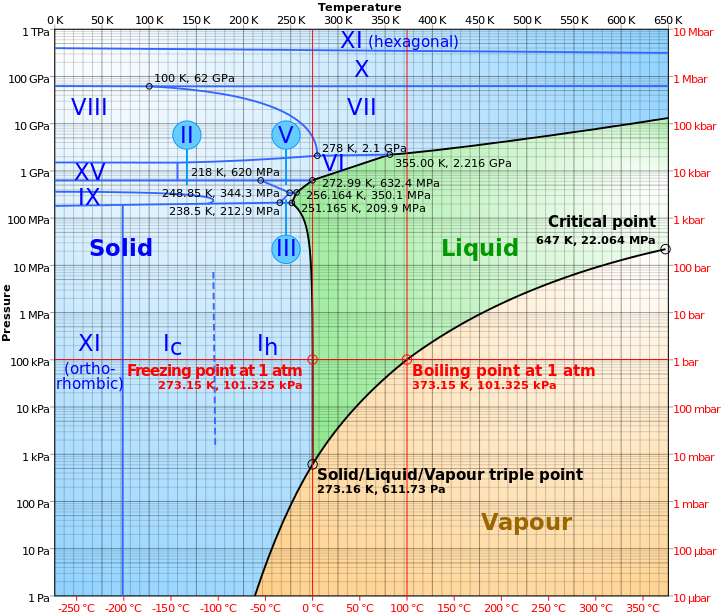
On the other hand, water has no business being a liquid at low temperatures, either. You can see — from this diagram below — that if you start with liquid water, you can turn it into a gas by lowering the pressure, but you can also turn it into a solid by lowering the temperature.
So my question is this:
If you took a glass of water into outer space, would the water freeze or would the water boil?
This is a question that seems awfully tough, because in addition to knowing all about water…

we also need to know about outer space. Space is a lot of things: cold, dark, and empty come to mind right away. And those things become pretty evident quickly, as soon as you leave the Earth.

Well, the temperature of space is, at its coldest, just the temperature of the leftover glow from the Big Bang. This radiation, known as the Cosmic Microwave Background, bathes the entire Universe in a temperature of only 2.7 Kelvin. If you can adequately shield yourself from the Sun, the Earth, and all other sources of heat in our local neighborhood, that’s how cold space is!
That temperature is less than 3 degrees above absolute zero, or -455 degrees Fahrenheit, so it’s vital when we send humans to space to keep both appropriate temperatures and pressures for them to survive. In a sealed environment like aboard the international space station, water behaves pretty similarly to how it does on Earth, with the remarkable exception of gravity.
But if you were to put some liquid water out into deep space with its freezing temperatures, would it freeze? Remember that there’s also — literally — zero pressure in space. So, what happens? Who wins? Does the water freeze from the low temperatures, or boil from the lack of pressure?
Oddly enough, the answer is first one, and then the other! Remember the phase diagram of water?
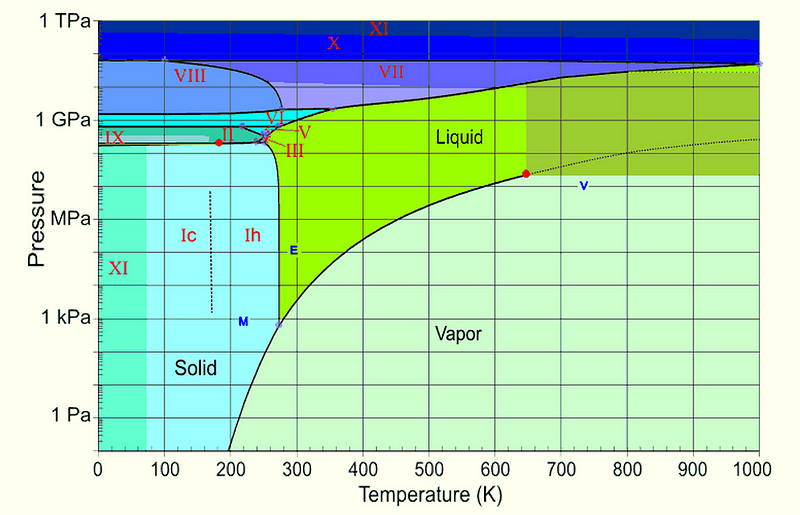
It turns out that having a pressure vacuum results in an incredibly rapid transition, causing the water to boil almost instantly. The (formerly) liquid water has no choice but to enter the gaseous phase, while it will take quite some time for its temperature to drop significantly enough to transition into the solid phase. In other words, the effect of boiling is much, much faster than the effect of freezing under these conditions.
But the story doesn’t end there. Once the water has boiled, we now have some isolated water molecules in a gaseous state, but a very, very cold environment! These tiny water vapor droplets now immediately freeze (or, technically, desublimate), and become ice crystals.

We’ve actually observed this before. According to astronaut observations, where they’ve observed their urine getting expelled from the spacecraft:
When the astronauts take a leak while on a mission and expel the result into space, it boils violently. The vapor then passes immediately into the solid state (a process known as desublimation), and you end up with a cloud of very fine crystals of frozen urine.
Sounds like it would be a fantastic thing to watch, doesn’t it? Well, many of you (recently!) have done almost the same thing on Earth. (Although some of you were not so successful.) What happens if you take boiling water and, on a very, very cold day, throw it up into the air?
The water’s surface area increases dramatically, where it finishes boiling almost immediately thanks to the molecules’ high speeds and becomes a gas. The gas then freezes (or desublimates) almost immediately, and ice crystals — a.k.a. snow — is the result!

And that’s what happens to water in space.
An earlier version of this post originally appeared on the old Starts With A Bang blog at Scienceblogs.





