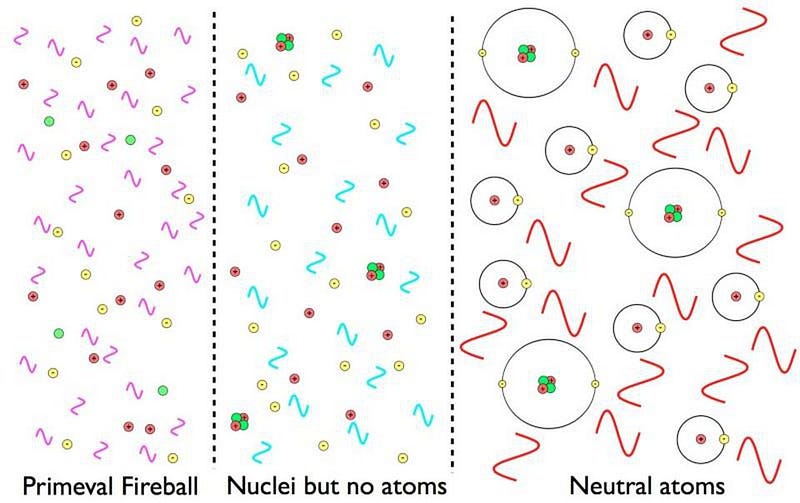
What Was It Like When The Universe First Made Atoms?
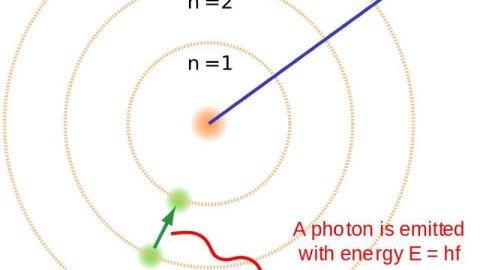
It took hundreds of thousands of years to make atoms for the first time. If things were just a little different, it could have taken an eternity.
When it comes to our world, our Solar System, and everything we can see in our Universe, it’s all made up of the same ingredients: atoms. Electrons and atomic nuclei interact and link up to form not just individual atoms, but simple and complex molecules, some of which have given rise to macroscopic structures and even life. It’s one of the most impressive facts about the Universe: that it exists in such a way to admit the complex structure we find within it today.
But for hundreds of thousands of years, dating from the instant of the hot Big Bang, it was impossible to form even a single atom. It took a huge amount of cosmic evolution, and a number of important steps, in order to create them. Here’s the story of how we got here.
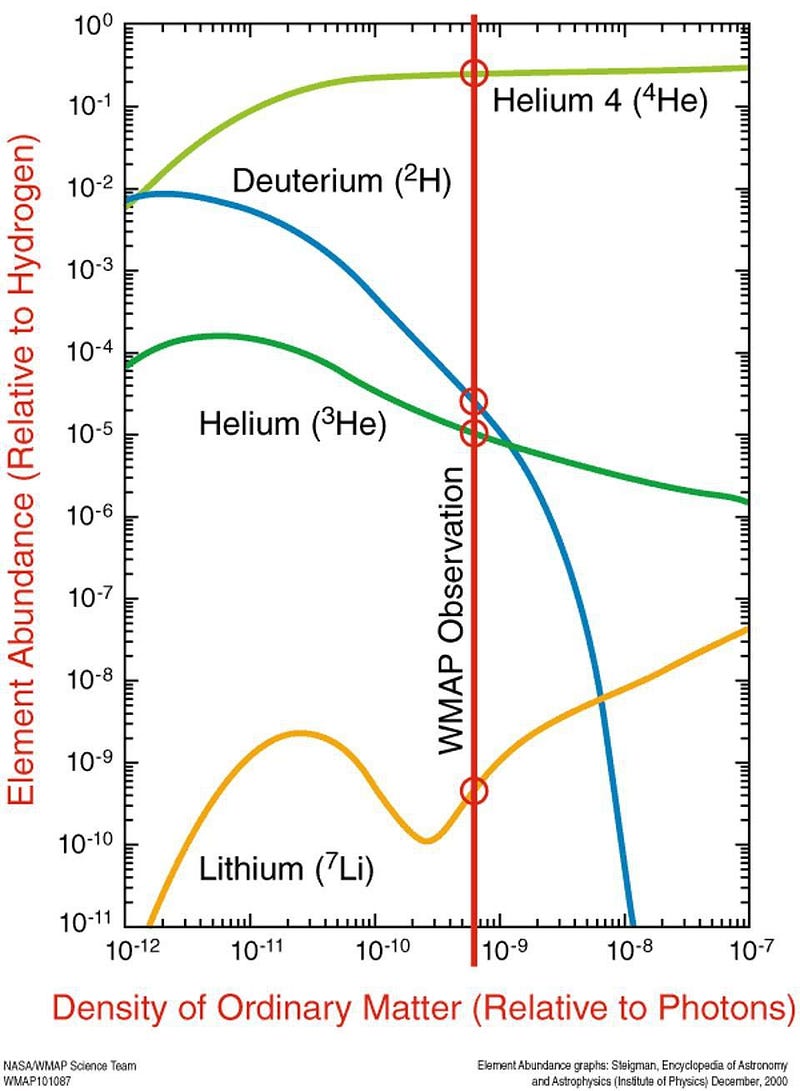
By time the Universe is four minutes old, it’s already done fusing all the atomic nuclei it can fuse in this hot, dense, early state. There are no more free neutrons; they’ve all been incorporated into heavier nuclei. These include:
- Helium-4 (two protons and two neutrons),
- Deuterium (one proton and neutron),
- Helium-3 (two protons and one neutron) and Tritium (one proton and two neutrons),
- and Lithium-7 (three protons and four neutrons) and Beryllium-7 (four protons and three neutrons).
That’s pretty much it. There are exactly enough free electrons to keep the Universe electrically neutral, balancing out the number of protons precisely. While photons, the particles that are the quanta of light, scatter off of both the electrons and the atomic nuclei continuously, it’s far too hot or energetic for anything else to form.
The reason for this is simple: there isn’t enough energy for these nuclei to fuse together into heavier combinations, but there’s too much energy for electrons to bind to them and form atoms. In fact, there’s way too much energy to form neutral atoms. By time the Universe is a few minutes old, the temperature is still hundreds of millions of degrees, but in order to form a stable, neutral atom, the temperature must drop below a few thousand degrees.
Sure, the Universe is expanding, which means its cooling as the wavelength of light within it stretches. But to stretch by that much — by a factor of about 100,000 — is going to take a lot of time.
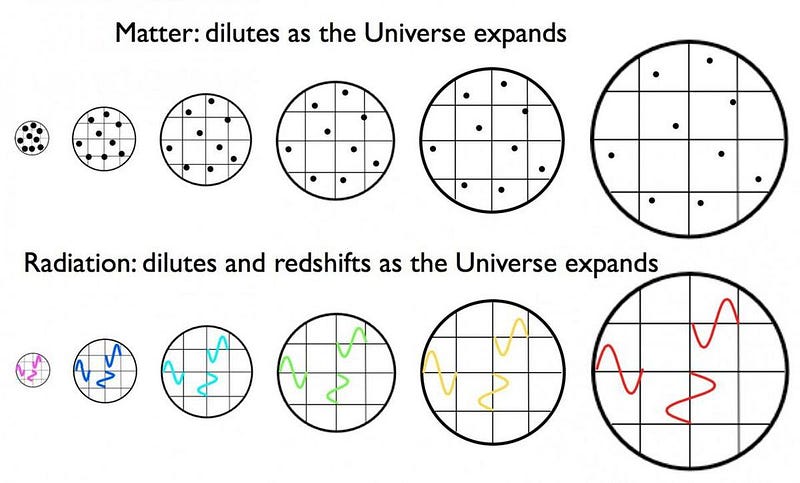
So the Universe waits. And as time goes on, it does expand and cool. As the minutes turn to hours and then to days, Beryllium-7 starts to radioactively decay. By capturing electrons, it slowly transforms its way into Lithium-7, and after a year or two, it’s virtually completely gone. As the years turn into decades, Tritium radioactively decays (by electron emission) into Helium-3. The transformation is complete after roughly a century.
And yet, still it’s far too hot to form a stable atom. So the Universe expands, cools, and gets less dense.
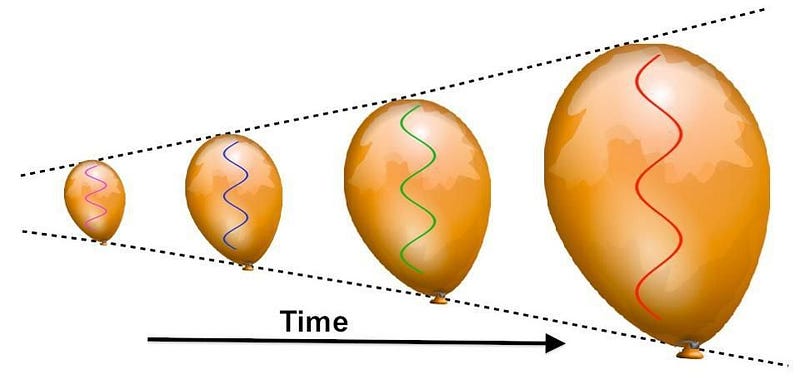
As the centuries turn into millennia, the redshifting of these photons — which outnumber the other particles by about a billion-to-one — becomes so severe that they’ve lost almost all of their energy. After a few tens of thousands of years, the radiation density drops below the matter density, meaning that the Universe is now dominated by slow-moving matter, rather than radiation which moves at the speed of light.
With this critical change, gravitation can pull the dark matter into clumps, which grow and grow, attracting more matter to them. Without radiation to wash these clumps out, the Universe begins to form structure. The seeds of our cosmic web have been planted.
But it’s still too hot to form neutral atoms. Every time an electron successfully binds with an atomic nucleus, it does two things:
- It emits an ultraviolet photon, because atomic transitions always cascade down in energy levels in a predictable fashion.
- It gets bombarded by other particles, including the billion-or-so photons that exist for every electron in the Universe.
And during these early stages, even when the Universe is tens of thousands of years old, there are enough photons with enough energy that pretty much as soon as an electron binds to a nucleus — whether a free proton or a heavier nucleus — it immediately gets blasted back apart.
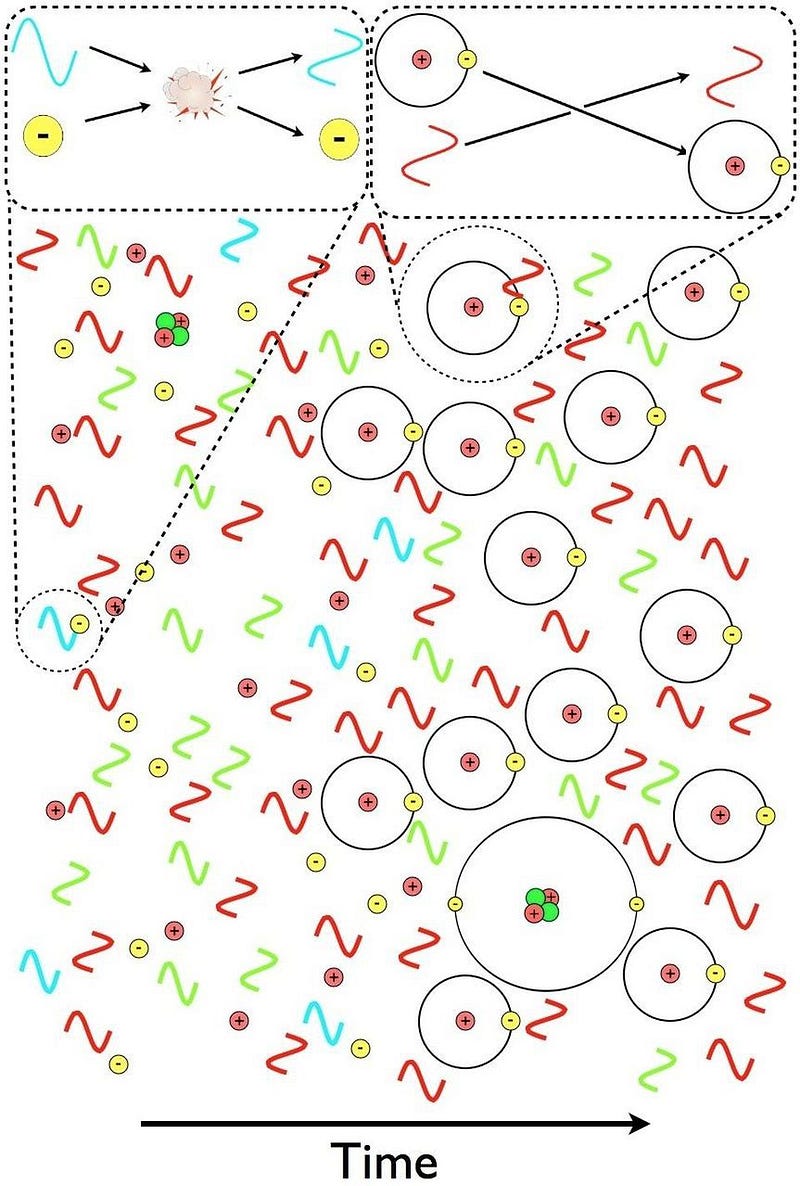
But something starts to change when the Universe reaches around 300,000 years of age. The background photons that are part of the leftover Big Bang are becoming too cool to immediately kick electrons off of their nuclei. There are still a few of those very high energies, but there are now fewer such photons than there are electrons in the Universe; less than 1-in-a-billion photons can ionize a neutral atom.
This means that neutral atoms can begin to form, but there’s a problem for them to remain. When you form a stable, neutral atom, they emit ultraviolet photons. Those photons then continue on, in a straight line, until they encounter another neutral atom, which they then ionize. Even though we can make a small number of neutral atoms, they don’t stay that way.
You might think that, eventually, these ultraviolet photons will travel through space for long enough that they’ll redshift, and no longer interact (because they’re not at the right wavelength) with the neutral atoms. That they won’t excite them anymore, leaving them incapable of ionization.
It’s true that this is an effect that happens, but it’s only responsible for a few percent of the neutral atoms that are first formed in the Universe. There’s another effect that comes in, instead, that dominates. It’s extremely rare, but given all the atoms in the Universe and the more-than-100,000 years it takes for atoms to finally and stably become neutral, it’s an incredible and intricate part of the story.
Most times, in a hydrogen atom, when you have an electron occupying the first excited state, it simply drops down to the lowest-energy state, emitting an ultraviolet photon of a specific energy: a Lyman alpha photon. But about 1 time in 100 million transitions, the drop-down will occur through a different path, instead emitting two lower-energy photons. This is known as a two-photon decay or transition, and is what is primarily responsible for the Universe becoming neutral.
When you emit a single photon, it almost always collides with another hydrogen atom, exciting it and eventually leading to its reionization. But when you emit two photons, it’s extraordinarily unlikely that both will hit an atom at the same time, meaning that you net one additional neutral atom.
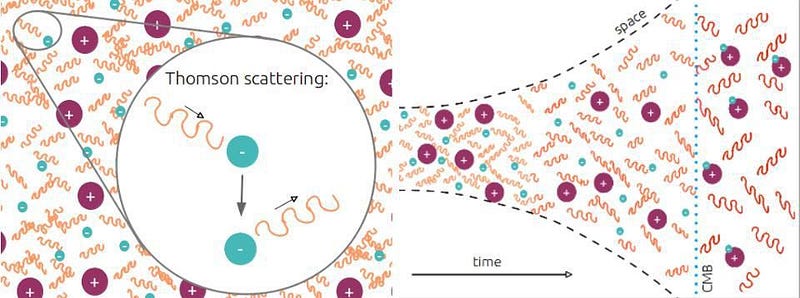
The rest is history. Sure, it takes more than 100,000 years for the process to complete, but this is how the Universe does it. This two-photon transition, rare though it is, is the process by which neutral atoms first form. It takes us from a hot, plasma-filled Universe to an almost-equally-hot Universe filled with 100% neutral atoms. Although we say that the Universe formed these atoms 380,000 years after the Big Bang, this was actually a slow, gradual process that took about 100,000 years on either side of that figure to complete. Once the atoms are neutral, there is nothing left for the Big Bang’s light to scatter off of. This is the origin of the CMB: the Cosmic Microwave Background.

We first detected this light beginning in 1964, confirming the Big Bang and ushering in the era of modern cosmology. From our best observations at present, we’ve been able to confirm this spectacular picture, even measuring the depth and thickness of the last-scattering surface from this time. Two-photon transitions have been verified here in laboratories on Earth, and what we’ve observed represents a spectacular agreement between our theoretical predictions and what actually occurred in the Universe’s distant past. It took around half-a-million years for the Universe to finally, completely form neutral atoms, all while gravitation began to pull the Universe together into clumps. The cosmic story that would lead to us, at last, was ready to continue on to the next phase.
Further reading on what the Universe was like when:
- What was it like when the Universe was inflating?
- What was it like when the Big Bang first began?
- What was it like when the Universe was at its hottest?
- What was it like when the Universe first created more matter than antimatter?
- What was it like when the Higgs gave mass to the Universe?
- What was it like when we first made protons and neutrons?
- What was it like when we lost the last of our antimatter?
- What was it like when the Universe made its first elements?