Why flammable ice could be the energy savior we’ve been looking for
- “Savior ice” is a form of flammable frozen gas called methane hydrate.
- Methane hydrate might one day serve as a bridge as we move away from fossil fuels.
- There is potentially more energy in methane hydrates than in all the world’s oil, coal, and gas combined. The primary challenge is harvesting it.
Now that humanity finds itself in an age of human-caused global warming, time will tell whether ice machines and polar ice can continue to coexist on the same planet.
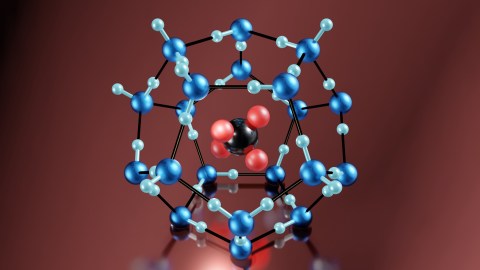
In the meantime, a great irony is at play. While the culture that ice created may be contributing to global warming, a very specific kind of ice may help wean Americans — and those in other countries — off fossil fuels. This “savior” ice, as it’s been called, is a form of frozen gas called methane hydrate, and it’s found throughout the Gulf of Mexico, off the coasts of Japan, and beneath Arctic permafrost. Methane hydrates are essentially balls of methane the size of a human hand encased in lace-like cages of ice that, when touched by a lit match, burst into flame — a property that has earned the substance the nickname “flammable ice.”
The science behind these balls of fire and ice is fascinating, but even more exciting is the possibility that they might one day serve as a bridge fuel.
Methane hydrates are formed through a combination of cold temperatures and relatively high pressure, and are found primarily on the edges of continental shelves, where land slopes sharply into the ocean. They also develop in and beneath permafrost in the Arctic, though they are less prevalent there.
When the pressure that formed them is lowered, or the temperatures are raised, they “disassociate,” meaning they separate into liquid water and methane gas, which can then, theoretically, be harnessed for energy. And hydrates hold a lot of potential energy. One cubic meter of the material can release up to 160 cubic meters of gas. There is potentially more energy in methane hydrates than in all the world’s oil, coal, and gas combined. Moreover, some scientists argue that they are safer to mine than conventional gas or oil.
To gain a better understanding of this fascinating substance, I called Carolyn Ruppel, a research geophysicist at the U.S. Geological Survey and the project chief for the USGS’s Gas Hydrates Project. “There are a few things about hydrates that are interesting,” she told me. For one, hydrates form at shallower depths in the ocean than oil, and so are “easier to access than conventional reservoirs” of the fossil fuels that people use now to heat their homes. Another benefit is that gas hydrates come in “a tiny, concentrated package,” she said. “So you get much more methane from one of those than from drilling for gas at the same depth.” Hydrates also release less carbon dioxide per unit of energy than coal or oil, making them a somewhat cleaner alternative to other fossil fuels. What’s more, the BBC has reported that it might be possible to pump CO2 out of the atmosphere and back into hydrates to replace the methane as it’s used. This could “provide an answer to the yet unsolved question of how to store this greenhouse gas safely.”
The Japanese government is among the biggest investors in this research, having spent millions of dollars to carry out a number of test projects to determine whether hydrates can be effectively harvested. India, China, and South Korea have also begun to experiment with extraction. In 2000, the United States launched national research and development programs on Alaska’s North Slope and offshore in the Gulf of Mexico to determine whether hydrates can be used to replenish or augment domestic fuel supplies. The process of discovery hasn’t been smooth, however.
One problem scientists continue to face is how to extract methane hydrates without breaking them down. Extraction causes the pressure that formed them to lessen, causing the hydrates to separate. To avoid this, the gas needs to be extracted from where the hydrates were formed. The Japanese government has funded research to explore this possibility. In 2013, its research team managed to produce gas from the hydrates by drilling into the seabed of the Nankai Trough, which is off the eastern coast of Japan’s main island. By lowering pressure on those hydrates, they were able to release and collect the gas for six days before sand entered the well and blocked the supply. A second test completed in 2017 was slightly more successful, running for twenty-four days without technical problems.
These tests suggest that hydrates might one day be a viable energy resource for Japan, but public reaction to the news has been mixed at best. While there has been some excitement about the possibility of Japan becoming energy independent, there has also been some concern that the breakdown of the hydrates could release a lot of methane gas into the ocean and then into the atmosphere, contributing to global warming. And it’s not just the Japanese that are worried. Environmentalists from all over the world have expressed concerns. An article in National Geographic referred to methane hydrates as “climate time bombs” whose “fuses are burning.”
The acidification likely to happen from hydrate breakdown is much less than that which is happening because of human activity.
A lot of this worry is unfounded, said Ruppel. Because of the way that water and gas intermingle, “there’s not going to be this freight train of methane emitted at the sea floor shooting into the atmosphere.” She explained this idea further in a 2017 paper written with John Kessler, a professor in the Department of Earth and Environmental Sciences at the University of Rochester. Most of the methane released from underwater hydrates will oxidize before it ever reaches the surface, never entering the atmosphere. “Unfortunately,” she said, “that image [of the runaway freight train] has been promulgated by a lot of people who really push this catastrophism issue. One thing John and I tried to say in [our paper] is, ‘Wait a second, guys. If you want to worry about the atmosphere, let’s focus on the huge amounts of CO2 that humans are putting in [it].’” Not that the released gases cause zero damage. “To be clear,” Ruppel said, “the methane that could be released in the ocean is not innocuous, because it acidifies the ocean — at least a little bit.” But even here, the acidification likely to happen from hydrate breakdown is much less than that which is happening because of human activity.
Research is also being done to determine the likelihood that hydrates can be extracted from permafrost, she told me. In collaboration with experts from Japan, the Department of Energy and the U.S. Geological Survey are studying hydrates in Alaska’s North Slope. The Arctic environment is quite different from marine environments, but the hydrate reservoirs in both are formed under similar pressure and temperature conditions. Those in the permafrost are just easier to get to.
I mentioned to Ruppel that some environmentalists are concerned that Arctic hydrates will become destabilized as the permafrost thaws, releasing CO2 into the air and further contributing to global warming. This, too, Ruppel said, is not as great of a danger as some have made it out to be. The hydrate resources there, she said, just aren’t that big and their potential contribution to greenhouse gases in the atmosphere is a tiny fraction of the planet’s total carbon budget.
When I asked her what, then, the biggest obstructions are to harnessing this energy, she said that first and foremost it’s a logistics issue. “Transport is difficult,” she said. Not unlike 19th-century businessman Frederic “Ice King” Tudor, who had to figure out the logistics of shipping ice for long distances at sea, experts still need to determine how best to get gas from the hydrates — or at least their gas emissions — to where they’ll be most useful. “For example, if Japan wants to use methane from hydrates,” Ruppel told me, “they might have to build a pipeline from land to the Nankai Trough, which is extraordinarily deep and in a place with bad current.”
Flammable ice may be one of the last new forms of fossil fuels to be extracted on a commercial scale.
Logistics aren’t any easier for the United States, which would have to get hydrates or their gases from the continental shelf or Alaska’s permafrost. At the time of writing, there isn’t much incentive to do that. Like Canada, the United States is experiencing a shale gas boom. “The general sense is that, if some countries’ backs are up against the wall in twenty years,” Ruppel said, “and they would have reasons for not using other sources or those sources are depleted, then these can be a potential backup plan.”
Of course, even as a backup plan, methane hydrates aren’t completely devoid of environmental issues. While hydrates are somewhat cleaner than other fossil fuels, they are still wrapped up in the same social, economic, and environmental problems as conventional gas and oil, and thus, they will likely only be used as bridge fuel. That means flammable ice may be one of the last new forms of fossil fuels to be extracted on a commercial scale — as well as one of only a few to be developed at a time when the end of fossil fuels is in sight.
Ice may have led to America’s obsession with cold, but in the form of methane hydrates, it may help us buy more time on a planet grown too hot.