How jelly-like bodies help sea creatures survive extreme conditions
- In Slime: A Natural History, Susanne Wedlich explores all things slime — from its role in science fiction and horror stories to its real-life roles in ecosystems across the planet.
- This book excerpt centers on how sea creatures utilize slime underwater.
- Slimes and gel-like structures can help marine life survive prey, extreme pressures, and other threats.
“‘…so that at 32 feet beneath the surface of the sea you would undergo a pressure of 97,500 lbs; at 320 feet, ten times that pressure; at 3200 feet, a hundred times that pressure; lastly, at 32,000 feet, a thousand times that pressure would be 97,500,000 lbs – that is to say, that you would be flattened as if you had been drawn from the plates of a hydraulic machine!’ ‘The devil!’ exclaimed Ned.”
—Jules Verne, Twenty Thousand Leagues Under the Sea
Nineteenth-century Victorian society was seized by a succession of unlikely crazes, one of which was a passion for ferns. It was the age of discovery and everyone wanted to decorate their homes with wondrous living pieces. Once the botanist Nathaniel Bagshaw Ward had developed a sealed glass container for living plants, everyone from the humble laborer to the aristocrat could indulge in ‘pteridomania’ – tending to, growing and studying ferns. The obsession went so far that some species were pushed to the edge of extinction, while elsewhere new hybrids emerged out of the contrived proximity of different species.
These tightly sealed glass cases also opened the public’s eyes to the previously unknown wonders of the oceans. The first scientific expeditions had brought up mysterious creatures from the deep such as unknown corals, crabs and sponges. They could hardly be studied in their natural habitats, but their new homes behind glass made observation possible, as Anna Thynne, the first person to keep sea creatures in an aquarium in London, successfully proved. She kept marine animals like stony corals alive for years, allowing them to thrive and even multiply. It was no easy undertaking, particularly for her staff. At least once a day, a maid was required to spend a quarter of an hour aerating seawater, which had been transported to London from the coast, by standing next to an open window and pouring it from one container into another.
Thynne published her findings with the help of Philip Henry Gosse, who would go on to write a popular manual on the new craze, introducing the hobby to the masses. People keen to keep abreast of the latest fashions could now enjoy a little piece of the underwater world in their own living rooms. The big cities followed suit and huge aquaria were built as public attractions, from London to Berlin to New York. But exotic wildlife proved tricky to domesticate. Back then, it was simply impossible to keep conditions in the aquaria constant, oxygenation remained an issue and sensitive creatures died off by the dozen.
Soon enough, the public lost its enthusiasm for aquatic exhibitions, which often saw unhappy sea life wasting away, bobbing up and down in murky water. Yet an interest in the oceans survived. It was the age of marine discovery, and HMS Challenger was leading the charge. Marine biologist Antje Boetius, of the Max Planck Institute for Marine Microbiology in Bremen and the Alfred Wegener Institute in Bremerhaven, and her father, the writer Henning Boetius, take stock of the Challenger expedition in their book The Dark Paradise. The voyage, lasting four years and covering nearly 70,000 miles, involved 734 deep-sea mapping explorations and 255 deep-sea temperature recordings, and the trawler nets were deployed 240 times, creating a first, if fuzzy, picture of the oceans and their currents. This included thousands of species of sea creatures hitherto unknown to science.
The findings finally put to bed the Abyssus or Azoic theory, which speculated that the deep ocean was a dead zone devoid of life below a depth of 550 metres. There were also, according to the Boetiuses, numerous ‘fantastic strokes of luck’, such as a reading on 23 March 1875, in which the lead line spooled down seemingly endlessly close to the Pacific island of Guam, only reaching the sea floor at a depth of 8,100 metres: ‘like they had discovered the gates of hell’. The spot was named Swire Deep after Herbert Swire, the sub-lieutenant on board. It is part of the Mariana Trench, an oceanic trench which is also home to the Challenger Deep and, at a depth of almost 11,000 metres, is the deepest place on the planet.
Deep underwater, eternal darkness reigns. Temperatures are close to freezing and the pressure is 1,000 times higher than at sea level. The region below 6,000 metres is known as the Hadal zone and is reminiscent of the kingdom of Hades, god of the Greek underworld. Animals should hardly be able to survive here, at least that was what we thought until scientists took their machines into the hellish depths for the first time. A few years ago, they encountered to their great surprise a creature that, like so many others, uses jelly-like materials to adapt to its marine habitat.
The hadal snailfish lives in the Hadal zone in the North-west Pacific, where it can be found swimming rather too busily to do its name justice. It is a member of the snailfish family Liparidae, of which several hundred species of various different colors are already known to science, many of them inhabiting the world’s deep-sea trenches. The species Pseudoliparis swirei – also named after Sub-lieutenant Swire – lives more than eight kilometres below sea level and holds the record for the world’s deepest-dwelling fish. It’s an astonishing feat for this lively, pink-bodied little creature; the water pressure at the point where the fish was found is equivalent to the weight of an elephant on a fingertip. How do these animals compensate for the significant pressure they are subject to in this habitat?
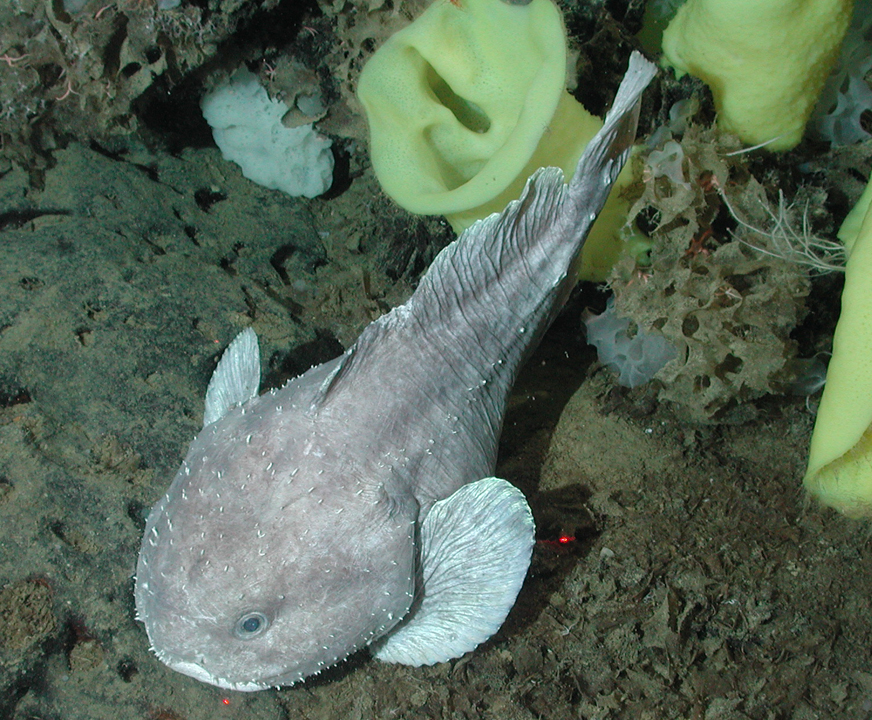
With its bulbous little body flowing into a flat, undulating tail, the scaleless snailfish looks like an oversized tadpole. It is faintly translucent, due to the gelatinous threads which run through its tissue. This jelly-like matrix helps it to withstand high pressure, improves buoyancy and probably makes it more streamlined. Many deep-sea fish produce gelatinous material of this kind, highly hydrated matter which requires little energy to construct while offering a quicker way to build up body mass than muscle would. This technique only works under pressure, however: if the snailfish is brought up out of the ocean depths, its tissue melts. The similarly gelatinous blobfish (Psychrolutes marcidus) was declared the world’s ugliest animal in 2013, even though its grumpy expression in a lumpen face was simply due to its collapsed tissue.
Here’s a curious side note: terrestrial organisms offer an unlikely champion of jelly-like structures in their tissues – plants. These gelatinous fibres or G-layers might have evolved with early land plants and are still widely used. The best-known example is that of trees which use gelatinous fibres in their aptly named tension wood to make sure their stems grow and keep upright while giving the branches a different orientation. Gelatinous fibres contain a sugary matrix and exhibit gel-like behavior like shrinking and swelling. This could in itself be a desirable function, since it conveys some flexibility to otherwise rather rigid plant structures like stems, branches, thorns and tendrils. Or even to whole plants: in some cases, these fibres pull entire shoots underground to survive fires or freezing.
But let’s return to the sea, where gel-like bodies are not confined to the deep ocean. Jellyfish, ctenophores, tunicates and many other animals – including planktonic larvae of myriad species – are composed largely of gelatinous matter. The bodies of jellyfish and comb jellies are made up of gel-like mesoglea, elastic fibres as well as muscle bundles and nerve fibres embedded in a highly hydrated matrix.
This is what makes the common jellyfish or moon jelly, Aurelia aurita, one of the ocean’s most effective swimmers, as the biophysicist John Dabiri of the California Institute of Technology was able to show. The animal’s bell pulsates, which rolls water off the top of it, creating a pull at its apex which the jellyfish uses to propel itself forward in a kind of suction movement, requiring no additional energy. A recent publication proved that the animals use another physical force to their advantage: when a plane lifts off or an animal swims close to a solid boundary, the so-called ‘ground effect’ gives them extra push. Jellyfish swim in open water without any natural surface in sight. But Aurelia aurita’s movements create a vortex in the water that acts like a ‘virtual wall’– making the master swimmer even better.
It’s an astonishing degree of efficiency for an animal that is made up of cheap biological material. The common jellyfish is little more than water, which offers a crucial advantage in the open ocean, though. These blue deserts, with no stony areas of shore, forests of kelp or other forms of hiding place, leave prey animals vulnerable if they don’t adapt to their environment by becoming invisible. Members of various groups have shifted to using gooey bodies because the material more or less reflects and bends light like its surrounding medium. It looks and behaves like water in the open ocean; it is, in other words, transparent. But not all body parts are capable of that. Eyes need to reflect light, and the digestive tract will be visible, at least when it’s filled. That is why one type of camouflage is not enough.
The hyperiid amphipod Cystisoma, a marine crustacean, for instance, can grow as long as a hand and is almost invisible. It helps that the animal has huge but only lightly tinted eyes because the dark, pigmented cells are spread over a large area. The trick works, as the biologist Karen Osborn at the Smithsonian Institution explains: ‘Mostly you see them because you don’t see them. When you pull up a net full of plankton, you see an empty spot – why is nothing there? You reach in and pull out a Cystisoma. It’s a firm cellophane bag, essentially.’
The glass squid goes even further. Its body is see-through but there are, again, those potentially treacherous eyes and the dark gut. Most predators approach from the depth and scan the water above them against the sky to find prey, but they will be hard-pressed to make out the squid. This time the animal seemingly fights fire with fire by illuminating its own eyes. However, this is no highlighting, but counter-illumination to hide any hard contrasts. That leaves the digestive gland as a problem to solve. This organ works a bit like our liver, is cigar-shaped and dark – and it can spin. As the squid moves around, the gland remains constantly upright, like a kind of biological compass needle. Hunters peeking up from the ocean’s depths, trying to find their prey, will have to spot the needle-like tip of the organ.
A couple of terrestrial species also make attempts to disappear into thin air, including the glass frog, whose camouflage is better described as translucent than transparent, according to a recent publication. This is not see-through invisibility but a softening of the edges, the blurring of a silhouette in order to meld visually into its surroundings. And there is a reason why gelatinous bodies give terrestrial animals away: highly hydrated jelly imitates water to perfection because it is not much more itself. But gelatinous bodies fail to imitate the less dense air, which bends and reflects light in a different way – which is always a giveaway. Even though the dream of invisibility is as old as humanity, living creatures will probably have to rely on optical tricks instead of actual see-through bodies since these would have to behave like air.
H.G. Wells must have pondered that problem a lot, preferring as he did to underpin his novels with solid science. In The Invisible Man he sets himself the task of describing the transparent body of the scientist Jack Griffin – the result of a failed experiment undertaken in a fit of hubris – in a manner both plausible and consistent, right up to the piece of cheese the scientist eats, which consequently makes its ‘ghostly’ way through his invisible digestive tract:
Is there such a thing as an invisible animal? . . . In the sea, yes. Thousands – millions. All the larvae, all the little nauplii and tornarias, all the microscopic things, the jelly-fish. In the sea there are more things invisible than visible! I never thought of that before. And in the ponds too! All those little pond-life things – specks of colourless translucent jelly! But in air? No!
Wells did a good job coming up with a sciencey explanation for his hero’s transformation that is completely unrealistic at the same time. True transparency will, for the time being, be the privilege of gelatinous animals in the sea, all but looking like water themselves. But see-through bodies aren’t the only tricks they have come up with to hide from predators. Slime can help in other ways too.
A slime-screen is one possibility. Some marine snails like the sea hare Aplysia emit purple clouds to ward off predators, with toxic ink as the main ingredient. The dark cloud is kept from diffusing right away by a good dose of mixed-in slime. Again, some squids go one better. If in danger, they add enough slime to their ink to create a pseudomorph. These are squid-shaped and squid-sized doppelgängers with only one job: to stay stable long enough to distract the predator. One species is even capable of creating a whole army, shooting out several pseudomorphs in a row, before blending in discreetly among its slimy comrades or slinking away.
But employing slime as a distraction doesn’t always have to be a matter of life and death. All the parrot fish really wants is a good night’s sleep out on the reef. Is that too much to ask? Without proper equipment it would be, but the brightly coloured animal simply secretes a slimy balloon to hide in. The head-to-tailfin sleeping bag is see-through but is thought to keep telltale molecular scents from escaping, which makes the fish all but invisible to parasitic Gnathiidae, the sea-dwelling equivalent of ticks.
Should these, or some other pest, latch on nonetheless, the unfortunate victim need only swim by a cleaner station in the coral reef. Big fish, turtles and even octopus can stop by to have dead skin and external parasites picked off by sharp-toothed cleaner fish. Mutual trust, or at least a truce of some sort, is essential because these little helpers work inside their clients’ open mouths, in between their sharp teeth. Yet it seems that the predators fall into a kind of trance which relaxes their biting reflex. This suits the cleaner fish too, because they’re able to snaffle small bites of nutritious slime as a treat off the skin of their daydreaming customers. It suits the bluestriped fangblenny even more, a mimic of a cleaner wrasse that only wants to get close enough to rip a mouthful of flesh out of an unsuspecting client, whose response to the attack will still be muted due to the parasite’s opiod-based venom.
Grabbing a bite of mucus or flesh is always a challenge, especially if your prey are stinging corals with a razor-sharp skeleton. The tubelip wrasse (Labropsis australis) has come up with an ingenious solution by giving a lubricated kiss of death. Its fleshy lips are arranged in fine folds, like the gills on a mushroom, and they are beset with goblet cells that make the mouth ooze with slime. That way the animal can suck the mucus and flesh of corals without feeling their stings or cut up its own delicate flesh. Another example where a fish’s soft anatomy is highly adapted to help handle prickly food concerns a type of wrasse that produces copious amounts of mucus in its mouth. Its diet consists mainly of gelatinous food – either organic waste or zooplankton – and the mucus might help to retain the slippery fare and neutralize any stinging cells.
But not all nutritious mucus must be fought for. Discus fish dispense their own slime willingly. Well, for a time at least. Both male and female parents allow their young to spend a month eating the rich gel from their skin, which is saturated with immune factors. However, as the weeks pass, the provisions cause conflict: the young latch on with greater frequency, with parents taking alternating shifts until they eventually go on strike. It’s a special way of caring for a brood; scientists consider the biparental mucus-feeding more similar to the habits of mammals and birds than of other fish. And it’s not the only example of cannibalistic offspring: caecilians are land-dwelling amphibians whose females allow their young repeatedly to gobble up the thick outer layer of their own skin.
But back to well-armed prey – and shelter: pearlfish hide in an unexpected place, as John Steinbeck observes in The Log from the Sea of Cortez:
In one of the sea cucumbers we found a small commensal fish, which lived well inside the anus. It moved in and out with great ease and speed, resting invariably head inward. In the pan we ejected this fish by a light pressure on the body of the cucumber, but it quickly returned and entered the anus again. The pale, colorless appearance of this fish seemed to indicate that it habitually lived there.
And they need their plentiful skin-slime as a lubricant when they slip into the sea cucumber’s rear end, which can’t be squeezed shut since these creatures breathe through their anuses. To add injury to insult, Encheliophis pearlfish not only use their hosts as refuges but also eat the sea cucumbers’ internal tissues. Yet the inside of a sea cucumber is not completely defenseless against attacks of all kinds. It can expel its thread-like and rather sticky intestines, which also secrete powerful toxins. This doesn’t make for a cozy shelter, but pearlfish somehow prevail by secreting an extra-thick slime coating for protection.
The pearlfish’s slimy sheath might be a unique feature in response to its special accommodation, but external mucus layers also help other fish to lubricate their way through the water and narrow openings. And these barriers possess many more important functions as the interface between the animal and its environment. We know that the mucus can contain antimicrobial and immune-related molecules to prevent infections while housing the microbiota. Fish mucus – which can be similar to our mucin-based hydrogels – has a social function as well. It helps with the communication between members of the same species to synchronize their spawning or coordinate shoaling, for example.
Communication is a double-edged sword, though, since it can lure unwanted suitors as well. The parasitic flatworm Entobdella soleae only attaches itself to the skin of the common sole, which their larvae must seek out and infest immediately after hatching. The nocturnal sole spends its days half buried in sediment, which makes it easier to target. This is probably why most attacks happen in the morning, but the larvae keep their schedule flexible. If they so much as catch a whiff of the slime the sole has left nearby or even on top of their eggs, they will hatch immediately.
Scientists have been trying to copy that feat of honing in on mucous markers. They often have a hard time detecting all species, especially the rare or hidden ones that live in aquatic ecosystems. But since sloughed-off mucus can contain cells of the organism it came from, all the scientists have to do now is screen water samples for genetic traces, the so-called environmental DNA. A similar method can be useful for checking the health of giant organisms. Scientists used to be reliant on skin and tissue samples to assess a whale’s health, but these were hard to get; now they use drones to catch the mucus that gets expelled whenever the animal breathes through its blowhole. It contains cells of the whale itself but also samples of the microbiota, and possibly pathogens.
Dangerous stowaways are a problem on their outer barriers as well. Many whales are routinely and visibly infested with parasites and other pests, which is a consequence of their unique evolutionary history. Unlike fish that never left the water, whales were adapted to life on land without an outer layer of mucus before they returned to the sea which makes it easier for parasites to attach. Pilot whales, however, have developed a very smooth skin which is self-cleaning. The spaces between their cells produce a kind of slime containing enzymes which fills in uneven spots and makes it harder for pests to gain a foothold.
But it’s an eternal arms race, and some parasites may in turn adapt to the new barrier and use it to find their host. Not all slime-loving larvae are a menace, however. The microscopic offspring of worms, mussels, corals, crustaceans, sponges and other marine animals float through the sea as plankton, looking for a good habitat. Since they settle down only once to metamorphose into their sessile adult forms, it has to be the perfect spot. Numerous environmental factors have a part to play in this process, which is critical for the survival of whole populations of marine invertebrates.
When the larvae choose their future homes one aspect stands out, which some scientists see as a universal mechanism. Larval settlement and metamorphoses could be induced – and possibly inhibited as well – by microbial slimes. These complex biofilms are ubiquitous and will quickly grow on any surface in seawater, often with different species of bacteria, unicellular algae and other microbes. It is difficult to unravel which specific signal sends which kind of message to induce or repel different invertebrate larvae, and we don’t know the details in most cases yet, but the connection itself is established. Larvae of the tubeworm Hydroides elegans, for example, will refuse to latch on if a biofilm is not in place, and even seem to prefer specific bacterial species.
If certain biofilms offer marine larvae ‘love at first taste’, as some scientists have called it, then sharks get all the feels from slime. Just like rays, these predators hunt with the help of sensory organs in the skin, known as ampullae of Lorenzini. Filled with jelly, these pores and channels pick up the tiniest changes in pressure. If an organism moves even slightly and at a great distance, the shark can locate it via its slimy antennae. If the search leads to a hagfish, though, the shark will end up with only a slimy gag for its trouble. Disappointment is also served up to the unlucky ray that risks a bite of the starfish Pteraster tesselatus: under attack, a hollow layer under its skin floods with enough repellent slime to spill over.
Another slime-emitting sea creature is the worm snail (Vermetidae). After they settle as larvae, the adult animals spend their whole lives in one spot in chalk tubes that look like either tightly wound or unraveled snail shells. That lifestyle poses two problems: how to feed? And how to reproduce? Slime is the answer to both questions. Like spiders in their webs, the worm snails let sticky lines float in the currents as traps for prey. From the opening of their tubes, they shoot slime nets into the open water that may even overlap like a web in colonies of the animals. These slimy shrouds can destroy coral tissues, which suggests that they may well contain toxic chemicals. When the time comes for reproduction, the males simply release their sperm bundles into the open water where they become trapped in the females’ nets, sticking to their slimy fishing lines before being reeled in.
In the dark and rather empty depth of the sea, however, females stuck in one place couldn’t risk having their sperm traps come in empty again and again. The worm Osedax mucofloris has had to find another way to secure the next generation. This bizarre animal lives on the sea floor absorbing the last nutrients and fats from bones, preferring the skeletons of whales that have sunk down after their deaths in a journey that can last for weeks. These whale-falls induce a kind of spring in the deep sea, where hundreds of species rely on the bounty from above, even if they’re not as specialized as Osedax is. The worms anchor themselves to the bone tissue using spurs, much like the roots of plants and covered in a mucus that dissolves the tissue or protects the animal amid the crumbling bones. But the whole animal is surrounded by a gelatinous tube which houses a harem of more than 100 dwarfish males.