An Alzheimer’s vaccine might be possible
- Alzheimer’s disease (AD) is thought to be caused by the accumulation of amyloid-beta proteins.
- There are no treatments that stop or prevent the disease, but scientists have now identified a possible lead.
- Antibodies against a portion of the amyloid-beta protein prevent memory loss in a mouse model of AD, implying a vaccine could be made.
Of the 2000 potential Alzheimer’s disease (AD) treatments explored over the last 30 years, fewer than ten have been shown to slow the development of symptoms, and none have been shown to cure or even stop the disease. However, a team of UK and German scientists has uncovered a novel approach that may not just stop AD but also prevent the disease from occurring in the first place.
The amyloid hypothesis
The cause of AD is unknown, but the amyloid hypothesis is the most widely accepted explanation. It posits that the disease develops as a type of protein (amyloid-beta) clumps together, forming aggregates in the brain. These aggregates cause neuron death, which ultimately results in cognitive decline. (It is believed that aggregation of the tau protein plays a role, as well.)
Three types of aggregates are considered when developing potential treatments. Two of the aggregates are soluble — small clumps of either shortamyloid proteins or longamyloid proteins that float around in the fluid that fills the spaces between neurons. Over time, these soluble aggregates become so large that they form the third type of aggregate: insoluble plaques composed of both short and long amyloid proteins that stick to the surface of neurons. All three have been implicated in neuron death and dysfunction. However, it is the plaques, a hallmark found in all AD patients, that scientists believed were the main cause of the disease.
However, the research team led by Mark Carr and Thomas Bayer was skeptical about the traditional approach, for good reason. First, the AD treatments that target amyloid proteins found in plaques do not significantly reduce symptoms. In fact, they can induce worse symptoms in some AD patients. Second, treatments that do not target the plaques often target soluble, longamyloid proteins. It is believed that long amyloid proteins are the main contributor to plaque formation, as they tend to aggregate into plaques more quickly than shortamyloid proteins. However, these long amyloid proteins seem to be a normal physiological product that is continuously generated in healthy individuals. While it is unclear what their function is, there is growing research that targeting them causes problems.
In a previous study, the team discovered an antibody that specifically targets short amyloid proteins. When mice with features of AD were treated with this antibody, neuron death stopped, and cognitive abilities improved. In other words, symptoms weren’t just slowed; they were reversed.
An anti-U-shaped antibody
Reversing symptoms of AD suggests the scientists uncovered a new mechanism of treatment. Since this antibody did not bind to long amyloid proteins or amyloid proteins in plaques, the researchers suspected that there might be something unique about the antibody-binding region on the short amyloid proteins. In the present study, they revealed the antibody binds to a region that folds back on itself, forming a U-shaped structure (called a hairpin). While this does not explain why the antibody was so successful at treating AD in mice, it has a huge implication: a vaccine could be made.
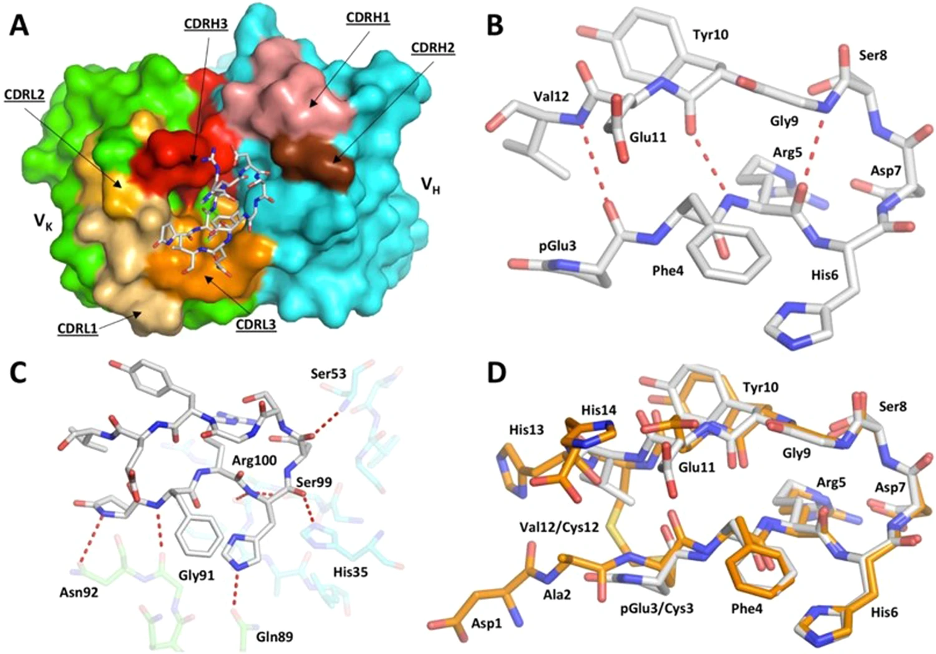
Carr explained: “This structure had never been seen before in amyloid beta [proteins]. However, discovering such a definite structure allowed the team to engineer this region of the protein to stabilize the hairpin shape and bind to the antibody in the same way. Our idea was that this engineered form of amyloid beta could potentially be used as a vaccine.”
In theory, vaccines are simple: introduce a foreign molecule into the body, and the immune system will start producing a lot of antibodies that recognize those molecules. However, in practice, vaccines are tricky. Most foreign molecules that enter your body are degraded by an arsenal of enzymes long before they can trigger antibody production. Thus, one of the most important parts of developing a vaccine is making sure the foreign molecule is stable enough to resist degradation. This can be surprisingly difficult. However, because a U-shaped structure loops around on itself, it is relatively easy to stabilize by “cyclizing” it. In other words, this U-shaped structure could be stabilized by changing it to an O-shaped structure. And if it can be stabilized, it can be turned into a vaccine.
Testing on a mouse model of AD
When the researchers immunized mice with AD-like traits with the cyclized vaccine, they observed a significant reduction in the amount of amyloid plaque present and neuron death, indicating that the vaccine halts the development of the disease, as well as the downstream impaired function.
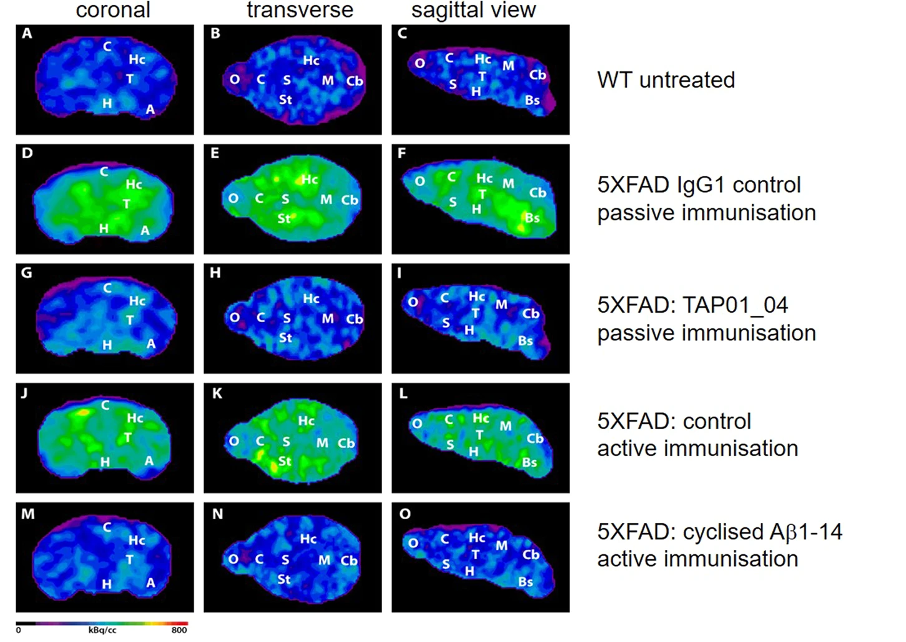
This led the researchers to ask if memory and cognition were also improved in immunized mice. They employed a method called the Morris water maze, in which mice are placed into a pool of water with a hidden platform. Healthy mice quickly learn where the platform is and can navigate to it, even from different starting positions. Mice with features of AD, on the other hand, always struggle to find the platform, suggesting a memory deficit. When these same mice were immunized with the cyclized vaccine, they were able to quickly navigate to the platform, suggesting the vaccine had prevented cognitive decline and memory loss.
Mark Carr added: “While the science is currently still at an early stage, if these results were to be replicated in human clinical trials, then it could be transformative. It opens up the possibility to not only treat Alzheimer’s once symptoms are detected, but also to potentially vaccinate against the disease before symptoms appear.”
The researchers hope to take the therapeutic antibody and the vaccine through clinical trials.